COMPANY ANNOUNCEMENT
Sanit Technologies Adds Label Clarification to Existing Voluntary Hand Sanitizer Recall
Some product packaging form labels are being added to ensure last stage of recall is complete
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionMicrobial contamination
- Company Name:
- Sanit Technologies LLC d/b/a Durisan
- Brand Name:
-
Brand Name(s)Durisan
- Product Description:
-
Product DescriptionHand sanitizer and hand sanitizing wipes
Company Announcement
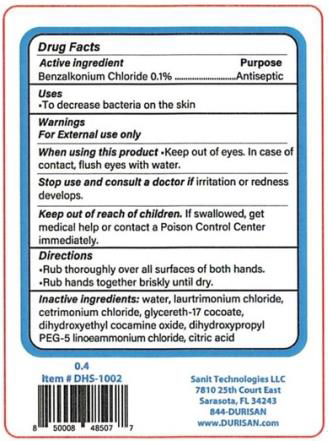
Sanit Technologies LLC d/b/a Durisan, is providing an additional 5 label illustrations and product sizes for customers that might not have been able to clearly identify containers of Non-Alcohol Hand Sanitizer products that were added to the voluntary recall initiated on March 24, 2021, and expanded by the April 10 and May 14, 2021 notices. To avoid any confusion, this press release provides copies of label illustrations for the recalled products.
Durisan Non Alcohol Antimicrobial Hand Sanitizer products became contaminated with a common municipal water supply borne bacteria, Burkholderia contaminans. Use of a hand sanitizer contaminated with Burkholderia contaminans, generally considered to be of low virulence, can range from no reaction to possible infections in a person with a hand wound or scrapes because the bacteria could enter the bloodstream, especially in patients with compromised immune systems.
To date, no qualified reports of adverse reactions have been reported related to this recall.
The product is intended to be applied topically to help reduce bacteria on the skin that could cause diseases when soap and water are not available.
The list of the lot numbers of the recalled products, which have a 24-month expiration, is included below. Consumers and customers in possession of Durisan Non Alcohol Antimicrobial Hand Sanitizer products with these numbers in any form factor are encouraged to contact Durisan to return it.
The product is packaged in sizes ranging from 18mL credit card to 1-gallon containers. The lot number can be found in black print on the bottom of the package.
Durisan Antimicrobial Solutions Hand Sanitizer Sizes With the Following Identification
|
Volume mL |
Volume oz |
UPC |
NDC |
|---|---|---|---|
| 18 | 0.61 | 8 52379 00614 1 | 71120-112-01 |
| 50 | 1.69 | 8 52379 00634 9 | 71120-611-20 |
| 118 | 4 | 8 52379 00612 9 | 71120-112-10 |
| 236 | 8 | 8 52379 00635 6 | 71120-112-11 |
| 250 | 8.45 | 8 52379 00611 0 | 71120-611-03 |
| 300 | 10 | 8 52379 00697 4 | 71120-112-08 |
| 550 | 18.59 | 8 52379 00620 2 | 71120-112-06 |
| 1000 | 33.81 | 8 52379 00610 3 | 71120-112-05 |
| 3785 | 128 | 8 52379 00621 9 | 71120-611-05 |
| 160 Ct Wipe | 5.63 | 8 52379 00631 8 | 71120-111-01 |
| 80 Ct Wipe | 2.81 | 8 52379 00632 5 | 71120-111-03 |
| 240 Ct Wipe | 8.44 | 8 52379 00633 2 | 71120-111-03 |
Durisan has provided written notification to its distributors and retailers and is alerting customers via this voluntary recall. Consumers that have the product are advised to contact Durisan to return it.
Consumers with questions regarding this recall can contact Durisan at 941-351-9114, 8:30 am - 4:30 pm Eastern time, Monday through Friday or by email at customerservice@durisan.com
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online by regular mail or by fax.
- Complete and submit the report OnlineExternal Link Disclaimer
- Regular Mail or Fax: Download formExternal Link Disclaimer or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Source: Sanit Technologies
| Lot Number | DHS051420A1-S |
| DHS041519A1-S | DHS051520A1R1-S |
| DHS041519A2-S | DHS052020A1-S |
| DHS041519A3-S | DHS052020A1R1-S |
| DHS041519A4-S | DHS052020B1-S |
| DHS041519A5-S | DHS052020B1R1-S |
| DHS041519A6-S | DHS052020C1-S |
| DHS042919AR1-S | DHS052020CR1-S |
| DHS043019AR1-S | DHS052220A1R1-S |
| DHS050319A4-S | DHS052220B1-S |
| DHS053019A1-S | DHS052620B1-S |
| DHS053019A2-S | DHS052720C1-S |
| DHS053019A4-S | DHS052720C1R1-S |
| DHS053019A5-S | DHS052720D1-S |
| DHS053019A6-S | DHS052820B1-S |
| DHS070219A1-S | DHS052820C1-S |
| DHS070219A2-S | DHS052820D1-S |
| DHS070219A3-S | DHS052920A1R1-S |
| DHS070219A4-S | DHS052920B1R1-S |
| DHS070219A5-S | DHS052920C1R1-S |
| DHS070219A6-S | DHS060120A1-S |
| DHS070219AB-S | DHS060220A1-S |
| DHS080219A1-S | DHS060320C1R1-S |
| DHS091819A1-S | DHS060520C1R1-S |
| DHS030920A1-S | DHS060520F1R1-S |
| DHS030920A2-S | DHS060820E1R1-S |
| DHS030920A3-S | DHS061220A1R1-S |
| DHS031020A4-S | DHS061920B1R1-S |
| DHS031020A5-S | DHS062220C-S |
| DHS031020A6-S | DHS062320B1R1-S |
| DHS031020A7-S | DHS062420B1R1-S |
| DHS031020A8-S | DHS081120A1-S |
| DHS031120A1-S | DHS081220A1R1-S |
| DHS031120A2-S | DHS081420B1-S |
| DHS031120A3-S | DHS081420B3-S |
| DHS031120A4-S | DHS081420B6-S |
| DHS031120A5-S | DHS081420B8-S |
| DHS031120A6-S | DHS081720A3-S |
| DHS032820B1-S | DHS081720A5-S |
Link to Original Press Release
Link to April 2021 Expansion
Link to May 2021 Expansion