COMPANY ANNOUNCEMENT
Durisan Hand Sanitizer Recall Due to Microbial Contamination
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionDue to microbial contamination
- Company Name:
- Sanit Technologies LLC d/b/a Durisan
- Brand Name:
-
Brand Name(s)Durisan
- Product Description:
-
Product DescriptionAntimicrobial Hand Sanitizer
Company Announcement
Sanit Technologies LLC d/b/a Durisan announces a voluntary recall of the lots listed in the table below of Durisan Antimicrobial Hand Sanitizer, Non-Alcohol products in various sizes listed. The products are being recalled due to microbial contamination. Specifically, out of specification results for bacterial count for Burkholderia cepacia complex and Ralstonia pickettii. The issue was discovered during a routine audit focused on production scale-up during the height of the pandemic.
|
Durisan Antimicrobial Solutions Hand Sanitizer Sizes With the Following Identification |
|||
|---|---|---|---|
| Volume mL | Volume oz. | UPC | NDC |
| 18 | 0.61 | 8 52379 00614 1 | 71120-112-01 |
| 118 | 4 | 8 52379 00634 9 | 71120-112-10 |
| 236 | 8 | 8 52379 00635 6 | 71120-112-11 |
| 300 | 10 | 8 52379 00697 4 | 71120-112-08 |
| 550 | 18.59 | 8 52379 00620 2 | 71120-112-06 |
| 1000 | 33.81 | 8 52379 00610 3 | 71120-112-05 |
Lot Numbers
| DHS030920A1-A | DHS051420A1-S |
| DHS030920A2-S | DHS051420A1-S |
| DHS030920A3-S | DHS052020B1-S |
| DHS031020A4-S | DHS052020C1-S |
| DHS031020A5-3 | DHS052220B1-S |
| DHS031020A6-S | DHS052620B1-S |
| DHS031020A7-S | DHS052720C1-S |
| DHS031020A8-S | DHS052720D1-S |
| DHS031120A1-S | DHS052820B1-S |
| DHS031120A2-S | DHS052820C1-S |
| DHS031120A3-S | DHS052820D1-S |
| DHS031120A4-S | DHS060120A1-S |
| DHS031120A5-S | DHS060220A1-S |
To date, no reports of adverse reactions or customer complaints have been reported related to this recall.
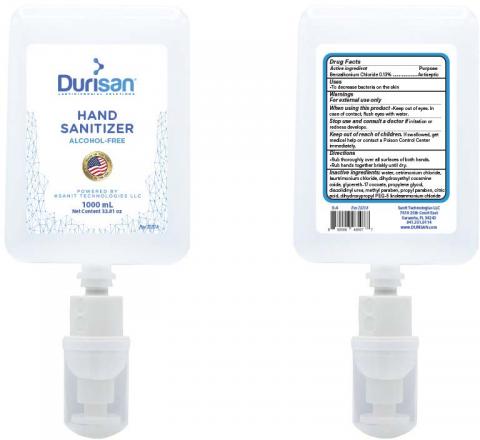
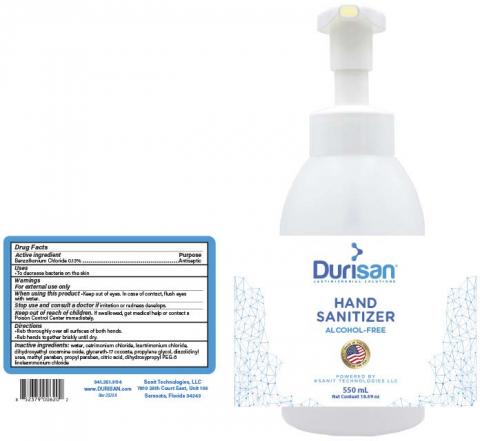
The product was packaged in sizes ranging from 18mL credit cards, to bottles sized in 118, 236, 300 and 550 mL and 1000mL wall mounted dispenser refills.
Use of a hand sanitizer contaminated with Burkholderia cepacia complex and Ralstonia pickettii, can range from no reaction to serious infections in a person with a hand wound or scrapes because the bacteria could enter the bloodstream, especially in patients with compromised immune systems. Health care professionals who use this contaminated hand sanitizer and tend to an at-risk patient, such as one with cystic fibrosis, could lead to adverse events ranging from a localized infection to lung or bloodstream infections, which could require patient hospitalization.
The product is intended to be applied topically to help reduce bacteria on the skin that could cause diseases when soap and water are not available. The product can be identified by examples of the product labels below. The product was manufactured from Feb. 1, 2020 until June 30, 2020, and distributed to selected retailers nationwide in the United States.
Durisan has provided written notification to its distributors and retailers and is alerting customers via this voluntary recall. Consumers that have the product which is being recalled are advised to destroy it immediately.
Consumers with questions regarding this recall can contact Durisan at 941-351-9114, 8:30 am - 4:30 pm Eastern time, Monday through Friday or by e-mail at customerservice@durisan.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Link to Expanded Release
Link to May 2021 Expansion
Link to June 2021 Expansion
Company Contact Information
- Consumers:
- Durisan
- 941-351-9114