COMPANY ANNOUNCEMENT
Roche Diagnostics to Replace CoaguChek® XS PT Test Strips
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Medical Devices
- Reason for Announcement:
-
Recall Reason DescriptionInaccurately high INR test results
- Company Name:
- Roche Diagnostics
- Brand Name:
-
Brand Name(s)Roche
- Product Description:
-
Product DescriptionCoaguChek XS PT Test Strips
Company Announcement
This notification supplements previous Urgent Medical Device Correction (UMDC) communications initially issued by Roche Diagnostics on September 12, 2018 and updated on October 17, 2018.
Roche Diagnostics will be proactively replacing all CoaguChek XS PT Test Strips in the United States.
- Patient Self-Testers (PST) will receive replacement test strips from their self-testing service providers.
- Professional / Healthcare Providers will receive their replacement test strips through Roche.
The affected CoaguChek XS PT Test Strips are used with the following CoaguChek professional and patient self-testing point-of-care meters listed in the table below.
| Healthcare Providers (professional) | Patient Self-Testers |
|---|---|
| CoaguChek XS Professional | CoaguChek XS PST |
| CoaguChek XS Pro | CoaguChek Vantus |
| CoaguChek XS Plus |
Roche Diagnostics, the manufacturer of CoaguChek meters and test strips, recently calibrated the CoaguChek XS PT Test Strips to the most recent International Normalized Ratio (INR) Standard. Since calibrating to this new standard, Roche Diagnostics has been informed of patients experiencing inaccurately high INR test results when testing with the affected lots of CoaguChek XS PT Test Strips listed in the table below.
| Product | Catalog numbers | Affected lot numbers | |
|---|---|---|---|
| CoaguChek XS PT Test 2x24 Strips |
04625315160 | 28124111 28124121 28631911 28631921 28631924 28632021 28632213 28632312 28632412 29415113 29415123 29494221 29494312 29494613 29494711 29778721 29779012 29779213 29779214 30497213 30497311 30497413 |
30497423 30497515 31404314 31404821 32264116 32264212 32264316 32264317 32264411 32264421 33045913 33046011 33046113 33046312 33046314 33046321 33046322 33449612 33449712 33449723 33449817 |
| CoaguChek XS PT Test 6 Strips |
04625374160 | ||
| CoaguChek XS Test 24 Tests USA |
07797826160 | ||
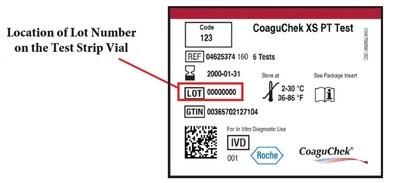
The lot number is printed on the test strip label, which is applied to the test strip vial. See the picture below for an example of the location of the lot number on the test strip vial.
Patients taking warfarin who receive inaccurate INR results above their target therapeutic range may be at risk for inappropriate therapeutic measures such as a warfarin dose reduction, interruption of warfarin use, or administration of vitamin K.
The affected test strips in the table above will be replaced with new CoaguChek XS PT Test Strips, which are not impacted by the Urgent Medical Device Correction (UMDC) and can be used without the need for confirmatory measurements with a laboratory method.
Action Required – Patient Self-Testers:
Stop using and discard any CoaguChek XS PT Test Strips listed in the table above. As of October 29, 2018, Roche started shipping newly calibrated test strips to healthcare providers and patient self-testing service providers. These test strips have been calibrated to the previous INR standard. For questions regarding when you will receive your new test strips, please contact your test strip provider. If you have any questions regarding your testing schedule, please contact your healthcare provider.
Action Required – Health Care Providers:
Stop using and discard any CoaguChek XS PT Test Strips listed in the table above. Also, please advise your self-testing patients to do the same (see above). Roche Diagnostics has started shipping unaffected test strips to healthcare providers, patient self-testing service providers and distribution partners.
Roche Diagnostics is notifying its healthcare providers, patient self-testing service providers, and distribution partner customers to arrange for replacement of all affected CoaguChek XS PT Test Strips listed in the table above. This will be executed through an updated Urgent Medical Device Correction (UMDC).
Healthcare providers and patients (self-testing customers) with questions may contact Roche Diagnostics Point of Care Technical Service by telephone at 1-800-428-4674.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people's lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalized healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world's largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. Thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Roche has been recognized as the Group Leader in sustainability within the Pharmaceuticals, Biotechnology & Life Sciences Industry nine years in a row by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2017 employed about 94,000 people worldwide. In 2017, Roche invested CHF 10.4 billion in R&D and posted sales of CHF 53.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit www.roche.com.
All trademarks used or mentioned in this release are protected by law.
For further information, please contact:
Nicole Clark
Communications Business Partner
Roche Diagnostics Corporation
(317) 361-9512
nicole.clark@roche.com
Company Contact Information
- Consumers:
- 800-428-4674
- Media:
- Nicole Clark
- 515-559-5770
- nicole.clark@roche.com