COMPANY ANNOUNCEMENT
Pfizer Expands Voluntary Nationwide Recall to include All Lots of CHANTIX® (Varenicline) Tablets Due to N-Nitroso Varenicline Content
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionN-nitroso-varenicline above acceptable daily intake level
- Company Name:
- Pfizer
- Brand Name:
-
Brand Name(s)CHANTIX
- Product Description:
-
Product DescriptionVarenicline tablets
Company Announcement
Pfizer is voluntarily recalling all lots of Chantix 0.5 mg and 1 mg Tablets to the patient (consumer/user) level due to the presence of a nitrosamine, N-nitroso-varenicline, at or above the FDA interim acceptable intake limit. As alternative suppliers have been approved in the United States, Pfizer is undertaking this precautionary measure.
Long-term ingestion of N-nitroso-varenicline may be associated with a theoretical potential increased cancer risk in humans, but there is no immediate risk to patients taking this medication. The health benefits of stopping smoking outweigh the theoretical potential cancer risk from the nitrosamine impurity in varenicline.
Nitrosamines are common in water and foods, including cured and grilled meats, dairy products and vegetables. Everyone is exposed to some level of nitrosamines. These impurities may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time.i
Chantix is a treatment to help patients quit smoking and is intended for short term use. People who smoke cigarettes are 15 to 30 times more likely to get lung cancer than people who do not smoke.ii
Smoking is also associated with many other cancers, as well as with cardiovascular disease and lung disease.iii CHANTIX has a safety profile that has been established over 15 years of marketing authorization and through a robust clinical program. Pfizer believes the benefit/risk profile of CHANTIX remains positive. Patients currently taking Chantix should consult with their healthcare provider about alternative treatment options. To date, Pfizer has not received reports of adverse events assessed to be related to this recall.
The NDC, Lot Number, Expiration Date, and Configuration details for Chantix Tablets are indicated in Appendix A. Photos of the products can be found in Appendix B. The products were distributed nationwide to Wholesalers and Distributors in the United States, US Virgin Islands and Puerto Rico from May 2019 to September 2021.
Pfizer places the utmost emphasis on patient safety and product quality at every step in the manufacturing and supply chain process. Pfizer has notified their direct consignees by letter to arrange for return of any recalled product.
Wholesalers and Distributors with an existing inventory of Chantix tablets, should stop use and distribution and quarantine the product immediately.
If you have further distributed the recalled product, please notify any accounts or additional locations which may have received the recalled product from you. Please conduct a sub-recall to those accounts and communicate this recall information immediately. Please request they immediately cease distribution of the product and promptly contact Stericycle at 888-276-6166 (Mon.-Fri. 8:00 am - 5:00 pm ET) to obtain a Business Reply Form (BRF) to initiate the return process.
If you received free product through the Pfizer Patient Assistance Program (PAP) or the Pfizer Institutional Patient Assistance Program (IPAP), please check your stock immediately. If you have any of the product in inventory, please follow the instructions above for returning the product to Stericycle Inc. Additionally, if you are aware of any patients to whom you dispensed the products and who still may have the product in their possession, please ask them to return the product to you and then follow the instructions above for returning the product to Stericycle Inc. For any questions related to Pfizer PAP or Pfizer IPAP product, please contact 833-203-2776 (Mon.-Fri. 8:00 am – 6:00 pm ET).
As communicated by FDA, there is no immediate risk to patients taking Chantix.iv Patients who are taking this product should consult with their health care provider to determine if alternate treatments are available. Patients with Chantix Tablets should contact Stericycle Inc. at 888-276-6166 (Mon.-Fri. 8:00 am - 5:00 pm ET) for instructions on how to return their product and obtain reimbursement for their cost.
Healthcare Professionals with questions regarding this recall can contact Pfizer using the below information.
| Contact Center | Contact Information | Area of Support |
|---|---|---|
| Pfizer Medical Information | 800-438-1985, option 3 (Mon.-Fri. 9 am-5 pm ET) www.pfizermedinfo.com |
For medical questions regarding the product |
| Pfizer Drug Safety | 800-438-1985, option 1 (24 hours a day; 7 days a week) | To report adverse events and product complaints |
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being executed with the knowledge of the U.S. Food and Drug Administration.
Appendix A: Recalled Product Details
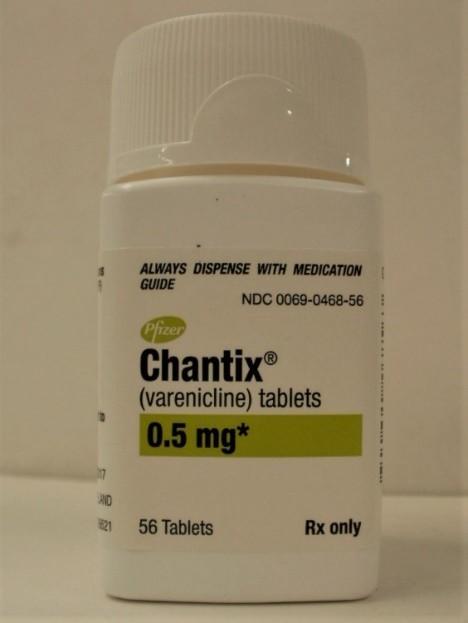
PRODUCT: Chantix Tablets, 0.5 mg NDC: 0069-0468-56
SIZE: Bottle of 56 Tablets
EXPIRATION DATE: January 2022 - May 2023
LOT NUMERS:
| 00019213 | DM9007 | EC6994 | EN8362 |
| CY6861 | DM9008 | EN5725 | EN8467 |
PRODUCT: Chantix Tablets, 1 mg NDC: 0069-0469-56
SIZE: Bottle of 56 Tablets
EXPIRATION DATE: September 2021 – December 2023
LOT NUMBERS:
| 00018777 | 00021024 | CW1572 | DF5280 | DY7987 | EN5694 |
| 00019289 | 00021073 | CW1573 | DF5281 | EA6080 | EN5695 |
| 00019593 | 00021074 | CW1574 | DF5282 | EC9841 | EP1717 |
| 00019682 | CW1565 | CW1575 | DR5086 | EC9842 | EP1718 |
| 00019846 | CW1566 | CW1578 | DR5092 | EC9843 | EP1719 |
| 00019977 | CW1567 | CW1579 | DR5093 | EC9847 | EW2012 |
| 00020295 | CW1568 | CW1581 | DR5094 | EC9848 | EW3854 |
| 00020448 | CW1569 | DF5277 | DT3885 | EE1011 | EW3865 |
| 00020458 | CW1570 | DF5278 | DW4148 | EM1069 | EX2102 |
| 00020480 | CW1571 | DF5279 | DW4152 | EM1070 | EX2103 |
PRODUCT: Chantix Tablets, 1 mg NDC: 0069-0469-03
SIZE: Carton containing 4 blister packs of 14 tablets each
EXPIRATION DATE: September 2021 – June 2023
LOT NUMBERS:
| 00019431 | 00021421 | 00022765 | DR2614 | DY7060 | EE9391 |
| 00019542 | 00021422 | 00022766 | DX4576 | DY9367 | EF2346 |
| 00019543 | 00021423 | 00023134 | DX5870 | DY9473 | EM4805 |
| 00019544 | 00022136 | 00023135 | DX5871 | DY9475 | EM4807 |
| 00020814 | 00022174 | 00023747 | DX5872 | DY9476 | EN2005 |
| 00020815 | 00022175 | 00023748 | DX5873 | DY9505 | ET1601 |
| 00020907 | 00022176 | DL3896 | DX7805 | EC5910 | ET1605 |
| 00020965 | 00022177 | DL7779 | DY6078 | EC5913 | ET1606 |
PRODUCT: Chantix Tablets, 0.5/1 mg NDC: 0069-0471-03
SIZE: Carton containing one blister pack of 11 0.5 mg tablets and one blister pack containing 42 1 mg tablets
EXPIRATION DATE: August 2021 – January 2023
LOT NUMBERS:
| 00018522 | 00020358 | 00021688 | 00022851 | DM0277 | ET1607 |
| 00018523 | 00020716 | 00021788 | 00023136 | DY4470 | ET1609 |
| 00018739 | 00020813 | 00021789 | 00023137 | EC5911 | ET1611 |
| 00018740 | 00021288 | 00021790 | 00023190 | EC5912 | |
| 00020231 | 00021289 | 00021791 | 00023448 | ED6814 | |
| 00020232 | 00021420 | 00021792 | DM0275 | ET1600 | |
| 00020357 | 00021687 | 00022819 | DM0276 | ET1603 |
References:
ii U.S. Centers for Disease Control and Prevention. What Are the Risk Factors for Lung Cancer?
https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm
Updated September 2020. Accessed June 2021.
iii U.S. Department of Health and Human Services. Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020.
Company Contact Information
- Consumers:
- Stericycle Inc.
- 888-276-6166
- Media:
- Eamonn Nolan
- 212-733-4626
- Eamonn.Nolan@pfizer.com