COMPANY ANNOUNCEMENT
Oscor Inc. Issues Recall of TB – Temporary Bipolar Pacing Lead (Unshrouded 2 mm Pins Models)
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Medical Devices
- Reason for Announcement:
-
Recall Reason DescriptionThe connector cap housing may slide and potentially expose the connection wire
- Company Name:
- Oscor Inc.
- Brand Name:
-
Brand Name(s)Oscor
- Product Description:
-
Product DescriptionTemporary Bipolar Pacing Leads
Company Announcement
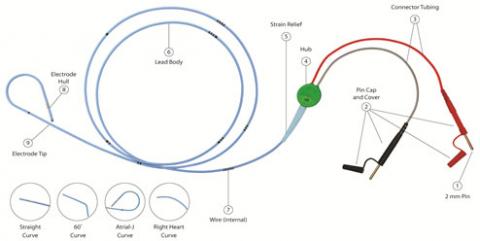
During the use of some TB - Temporary Bipolar Pacing Leads, featuring the 2mm unshrouded connectors, the connector cap housing (see Picture 1, No. 2 Pin Cap and Cover) may slide and potentially expose the connection wire. In some instances, this may cause the wire to be more susceptible to loss of connectivity or breakage during movement of the cables causing interruption of the pacing system. The analysis of the returned devices attributed the failure to a design change of the cap housing of the pins. In the last six years, a total of four serious injuries were reported to Oscor which were attributed to a connector cap malfunction causing the lead connector to separate during use potentially leading to an interruption of the pacing system. No deaths were reported; however the risk for possible injury is a concern if the connectors separates during use.
MODEL NUMBERS:
| GTIN | GUDID/Label Model Number |
TB Specification Description | |||
|---|---|---|---|---|---|
| Series | French size | Pin | Curve Type | ||
| 00836559009726 | 020004 | TB | 4F | Unshrouded | Straight |
| 00836559009733 | 020005 | TB | 5F | Unshrouded | Straight |
| 00836559009740 | 020006 | TB | 6F | Unshrouded | Straight |
| 00836559009788 | 020010 | TB | 4F | Unshrouded | Atrial J |
| 00836559009795 | 020011 | TB | 5F | Unshrouded | Atrial J |
| 00836559009801 | 020012 | TB | 6F | Unshrouded | Atrial J |
| 00836559009856 | 020017 | TB | 5F | Unshrouded | 60° Curve |
| 00836559009863 | 020018 | TB | 6F | Unshrouded | 60° Curve |
| 00836559009900 | 020022 | TB | 4F | Unshrouded | Right Heart |
| 00836559009917 | 020023 | TB | 5F | Unshrouded | Right Heart |
| 00836559009924 | 020024 | TB | 6F | Unshrouded | Right Heart |
| 00836559009030 | TBK04110USG | TB | 4F | Unshrouded | Straight |
| 00836559009054 | TBK05110USG | TB | 5F | Unshrouded | Straight |
| 00836559009078 | TBK06110USG | TB | 6F | Unshrouded | Straight |
| 00885672007027 | TBVK04110USG | TB | 4F | Unshrouded | 60° Curve |
| 00885672007034 | TBJK04110USG | TB | 4F | Unshrouded | Atrial J |
| 00885672004378 | TBRHK04110USG | TB | 4F | Unshrouded | Right Heart |
| 00885672103682 | TBRHK06110USG | TB | 6F | Unshrouded | Right Heart |
Table 1: TB-Temporary Bipolar Pacing Lead with Un-shrouded Pins Affected Models.
Warning:
For pacing dependent patients, an interruption of pacing system could result in serious injury or death if not detected. Continuous monitoring is required.
Customer may contact Oscor’s Customer Relations Group, Monday to Friday from 8:30AM to 5:30PM Eastern Time at 727-937-2511 or via email at TB@oscor.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.