COMPANY ANNOUNCEMENT
Lupin Pharmaceuticals, Inc. Issues Voluntarily Nationwide Recall of All Irbesartan Tablets and Irbesartan and Hydrochlorothiazide Tablets Due to Potential Presence of N-nitrosoirbesartan Impurity
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionAPI batches above the specification limit for the impurity, N-nitrosoirbesartan
- Company Name:
- Lupin Pharmaceuticals, Inc.
- Brand Name:
-
Brand Name(s)Lupin
- Product Description:
-
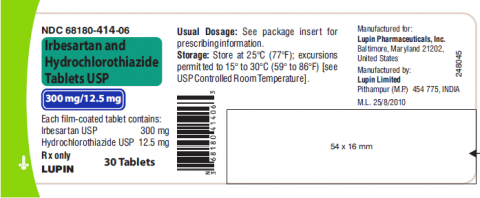
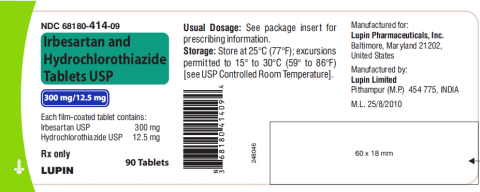
Product DescriptionIrbesartan and Hydrochlorothiazide Tablets USP, 150mg/12.5 mg and 300mg/12.5 mg
Company Announcement
Baltimore, Maryland, October 14, 2021: Lupin Pharmaceuticals Inc. is voluntarily recalling the below-mentioned batches of Irbesartan Tablets and Irbesartan and Hydrochlorothiazide Tablets to the consumer level. As part of Lupin’s ongoing assessment, analysis revealed that certain tested API batches (but not finished product batches) were above the specification limit for the impurity, N-nitrosoirbesartan. Although Lupin has received no reports of illness that appear to relate to this issue, the company, out of an abundance of caution, is recalling all batches of Irbesartan Tablets USP 75mg, 150mg and 300mg and Irbesartan and Hydrochlorothiazide Tablets USP, 150mg/12.5mg and 300mg/12.5mg in the US.
Lupin discontinued the marketing of Irbesartan and Irbesartan and HCTZ tabs in Jan 2021.
Risk Statement: N-nitrosoirbesartan impurity is a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests.
From October 8, 2018 (the earliest date of shipment from the manufacturing site of any of the affected batches), to September 30, 2021, Lupin received 4 reports of illness from Irbesartan and 0 reports from Irbesartan and Hydrochlorothiazide.
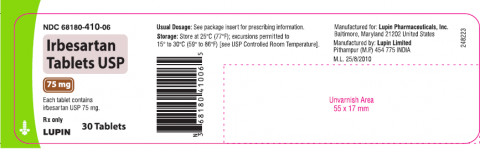
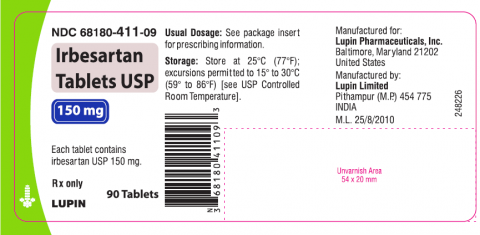
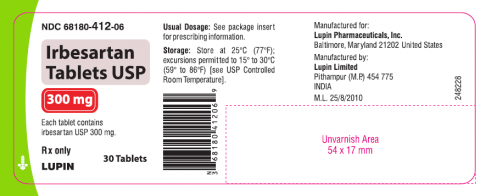
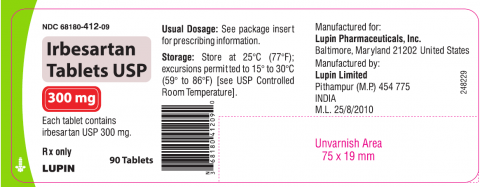
Irbesartan tablet USP is an angiotensin II receptor blocker indicated for treatment of hypertension, to lower blood pressure, diabetic nephropathy in hypertensive patients with type 2 diabetes, an elevated serum creatinine, and proteinuria. Irbesartan Tablets USP 75mg, 150mg and 300mg is packaged in 30 and 90 count bottles and was distributed nationwide in the US to wholesalers, drug chains, mail order pharmacies and supermarkets. Lupin discontinued the marketing of Irbesartan Tablets on Jan 7, 2021. The recalled lots are included in the table below:
|
Product |
Lot No |
NDC |
Distribution Dates |
|---|---|---|---|
|
Irbesartan Tablets USP, 75mg
|
H000843, H805727, H901579
|
68180-410-06 (30’s |
10/20/2018
|
|
H000844, H000964, H804311,
|
68180-410-09 (90’s | ||
|
Irbesartan Tablets USP, 150mg
|
H804403, H805251, H805640,
|
68180-411-06 (30’s | |
|
H804492, H805252, H805253,
|
68180-411-09 (90’s | ||
|
Irbesartan Tablets USP, 300mg
|
H804310, H900050, H902262
|
68180-412-06 (30’s | |
|
H000845, H000846, H000965,
|
68180-412-09 (90’s |
Irbesartan and hydrochlorothiazide tablet USP is a combination of irbesartan, an angiotensin II receptor antagonist, and hydrochlorothiazide, a thiazide diuretic, indicated for hypertension in patients not adequately controlled with monotherapy or as an initial therapy in patients likely to need multiple drugs to achieve their blood pressure goals. Irbesartan and hydrochlorothiazide tablet USP, 150mg/12.5mg and 300mg/12.5mg is packaged in 30 and 90 count bottles and was distributed nationwide in the US to wholesalers, drug chains, mail order pharmacies and supermarkets. Lupin discontinued the marketing of Irbesartan and HCTZ Tablets on Jan 7, 2021. The recalled lots are included in the table below:
|
Product |
Lot No |
NDC |
Distribution Dates |
|---|---|---|---|
|
Irbesartan and
|
H804537, H805148,
|
68180-413-06 (30’s |
10/17/2018
|
|
H000963, H804507,
|
68180-413-09 (90’s | ||
|
Irbesartan and
|
H804192, H805348,
|
68180-414-06 (30’s | |
|
H804082, H804121,
|
68180-414-09 (90’s |
Lupin Pharmaceuticals Inc. is notifying its wholesalers, distributors, drug chains, mail order pharmacies and supermarkets by phone and through recall notification and is arranging for the return of all the recalled product lots.
Patients taking, Irbesartan Tablets USP, 75mg, 150mg and 300mg and Irbesartan and Hydrochlorothiazide Tablets USP, 150mg/12.5mg and 300mg/12.5mg are advised to continue taking their medication and contact their pharmacist, physician, or medical provider for advice regarding an alternative treatment.
Wholesalers, distributors and retailers that have Irbesartan Tablets USP, 75mg, 150mg and 300mg and Irbesartan and Hydrochlorothiazide Tablets USP, 150mg/12.5mg and 300mg/12.5mg that are being recalled should discontinue distribution of the recalled product lots immediately and return it to Inmar Rx Solutions, Inc., 635 Vine St, Winston Salem, NC 27101. Tel: (855) 769-3988 / (855) 769-3989.
Consumers, wholesalers, distributors, and retailers with questions regarding this recall should contact Inmar Rx Solutions, Inc. at (855) 769-3988 / (855) 769-3989 Monday – Friday 09:00 am to 05:00 pm EST. For reimbursement, please have the recalled lots returned to Inmar Rx Solutions, Inc.; the lot number can be found on the side of the bottle label.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Inmar Rx Solutions, Inc.
- (855) 769-3988 / (855) 769-3989
- Media:
- Shweta Munjal
- shwetamunjal@lupin.com