COMPANY ANNOUNCEMENT
Janssen Issues Voluntary Nationwide Recall for One Lot of ORTHO-NOVUM 1/35 and Two Lots of ORTHO-NOVUM 7/7/7 Due to Incorrect Veridate Dispenser Instructions
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionDue to Incorrect Veridate Dispenser Instructions
- Company Name:
- Janssen Pharmaceuticals, Inc.
- Brand Name:
-
Brand Name(s)ORTHO-NOVUM
- Product Description:
-
Product DescriptionORTHO-NOVUM 1/35 and 7/7/7
Company Announcement
The ORTHO-NOVUM® product itself remains safe and effective for use with the appropriate dispenser instructions
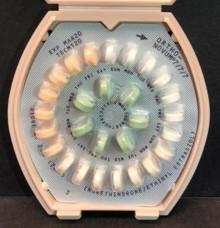
TITUSVILLE, NJ – Janssen Pharmaceuticals, Inc. has initiated a voluntary recall of one lot of ORTHO-NOVUM® 1/35 (norethindrone/ethinyl estradiol) Tablets and two lots of ORTHO-NOVUM® 7/7/7 (norethindrone/ethinyl estradiol) Tablets to the pharmacy level. The patient information provided inside affected packages of ORTHO-NOVUM® does not include the appropriate instructions for the Veridate® dispenser.
The potential risk of taking ORTHO-NOVUM® without the appropriate instructions for correct use of the Veridate® dispenser pack is that the consumer could take the pills in the incorrect order (still receiving an effective dose) or could take an inactive "reminder" pill instead of an "active" pill which could lead to breakthrough bleeding or an unintended pregnancy.
ORTHO-NOVUM® 1/35 and ORTHO-NOVUM® 7/7/7 tablets are indicated for the prevention of pregnancy in women who elect to use this product as a method of contraception.
The ORTHO-NOVUM® product itself remains safe and effective for use with the appropriate dispenser instructions. Women should continue to take the 21 "active" pills (with hormones) (peach for ORTHO-NOVUM® 1/35; white, light-peach and peach for ORTHO-NOVUM® 7/7/7) for three weeks, followed by the one week of green "reminder" pills (without hormones).
The three lots affected by this recall are:
|
Product Description |
NDC Number (carton) |
NDC Number (pouch) |
Lot No. |
Expiration Date |
|---|---|---|---|---|
| ORTHO-NOVUM® 1/35 | 50458-176-06 | 50458-176-28 | 18BM114 | 03/2020 |
| ORTHO-NOVUM® 7/7/7 | 50458-178-06 | 50458-178-28 | 18CM120 | 03/2020 |
| ORTHO-NOVUM® 7/7/7 | 50458-178-12 | 50458-178-12 | 18BM110 | 03/2020 |
This recall only affects the U.S., and no other ORTHO® contraceptive products beyond the three lots listed above are impacted by this recall action. ORTHO TRI-CYCLEN LO®, ORTHO TRI-CYCLEN®, ORTHO MICRONOR® and ORTHO CYCLEN® are not affected.
Products were distributed in the U.S. to wholesalers, distributors and pharmacies, and they have been notified by recall letter to return affected products.
Consumers with ORTHO-NOVUM® product from the affected lots can access the correct instructions for the Veridate® dispenser pack at https://www.janssen.com/us/our-products and talk to their prescribing medical professional if they have any concerns. Consumers should not stop taking the product and if they do miss a dose, they should follow the instructions included in the packet.
Consumers with questions regarding this recall can contact Janssen via phone on: 1-800-526-7736 (1-800-JANSSEN) Monday through Friday from 9:00 am to 8:00 pm ET. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About the Janssen Pharmaceutical Companies
Janssen Pharmaceuticals, Inc. is part of the Janssen Pharmaceutical Companies of Johnson & Johnson.
Learn more at www.janssen.com.