COMPANY ANNOUNCEMENT
IntegraDose Compounding Services, LLC Issues Voluntary Nationwide Recall of Cefazolin Injection Products Due to a Lack of Sterility Assurance
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
Pharmaceutical Quality - Reason for Announcement:
-
Recall Reason DescriptionLack of sterility assurance.
- Company Name:
- IntegraDose Compounding Services, LLC
- Brand Name:
-
Brand Name(s)IntegraDose Compounding Services, LLC
- Product Description:
-
Product DescriptionCefazolin
Company Announcement
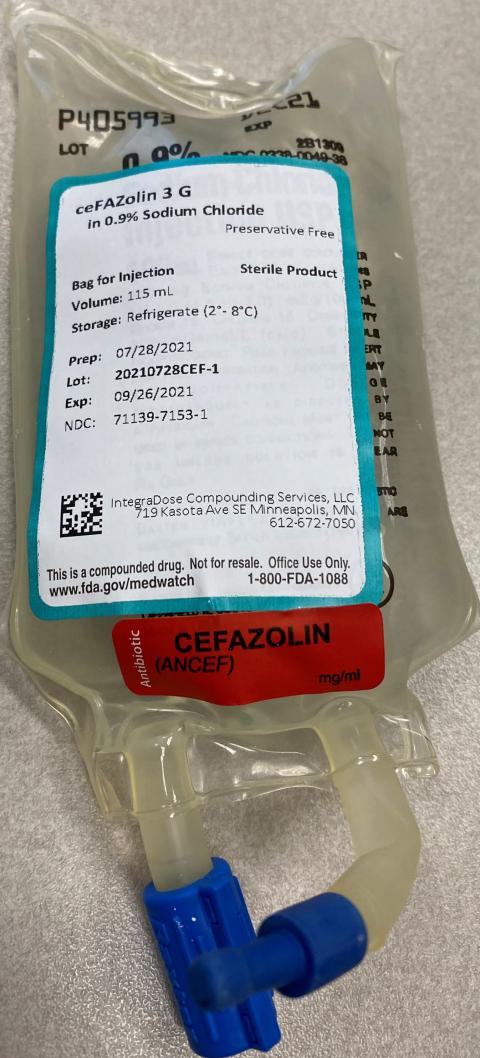
Minneapolis, Minnesota, IntegraDose Compounding Services is voluntarily recalling nine lots, listed in the table below, of cefazolin 2 gram in 20 mL syringe for injection and two lots of cefazolin 3 gram in 100 mL 0.9% sodium chloride bag for injection due to a lack of sterility assurance resulting from compounding in a newly installed biologic safety cabinet without completing dynamic smoke study testing.
|
Product |
NDC |
Lot |
Exp |
|---|---|---|---|
|
Cefazolin 2 gram in 20 mL syringe for injection
|
71139-7087-1 | 20210803CEF-1 | 09/17/2021 |
| 71139-7087-1 | 20210805CEF-3 | 09/19/2021 | |
| 71139-7087-1 | 20210806CEF-1 | 09/20/2021 | |
| 71139-7087-1 | 20210806CEF-2 | 09/20/2021 | |
| 71139-7087-1 | 20210809CEF-1 | 09/23/2021 | |
| 71139-7087-1 | 20210809CEF-2 | 09/23/2021 | |
| 71139-7087-1 | 20210810CEF-1 | 09/24/2021 | |
| 71139-7087-1 | 20210811CEF-1 | 09/25/2021 | |
| 71139-7087-1 | 20210812CEF-1 | 09/26/2021 | |
| Cefazolin 3 gram in 100 mL 0.9% sodium chloride bag for injection | 71139-7053-1 | 20210722CEF-2 | 09/20/2021 |
| 71139-7053-1 | 20210728CEF-1 | 09/26/2021 |
Intravenous administration of a non-sterile drug could result in serious infections ranging from fever, chills, and malaise, to severe adverse events such as septicemia, bacterial meningitides and wound infection which may be life-threatening. The possibility of a breach in sterility assurance in distributed product, while not confirmed, cannot be eliminated. No batches of product have been identified as containing microorganisms. To date, IntegraDose Compounding Services has not received reports of any adverse events associated with this issue for these lots.
Cefazolin is an antibiotic and the products are packaged in zip-locking bags containing ten units. The lots were distributed nationwide in the USA to hospitals from 8/12/21 to 9/15/21. IntegraDose Compounding Services has initiated an investigation to determine the root cause and corrective and preventative actions.
IntegraDose Compounding Services has notified its direct customers via a recall letter and is arranging for impacted product to be returned. Anyone with an existing inventory of the recalled lots should stop use and distribution and quarantine immediately.
Consumers with questions regarding this recall can contact IntegraDose Compounding Services by phone at (612-672-5216) or e-mail (celse1@fairview.org) on Monday - Friday, 8:00 AM – 4:00 PM CDT.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- IntegraDose
- 612-672-5216
- celse1@fairview.org
- Media:
- Craig Else, Director/COO
- 612-672-5216
- craig.else@integradose.org