COMPANY ANNOUNCEMENT
Innoveix Pharmaceuticals, Inc. Issues Voluntary Recall of All Sterile Compounded Drug Products Due to A Lack of Sterility Assurance
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionPotential lack of sterility assurance
- Company Name:
- Innoveix Pharmaceuticals, Inc.
- Brand Name:
-
Brand Name(s)Innoveix Pharmaceuticals, Inc.
- Product Description:
-
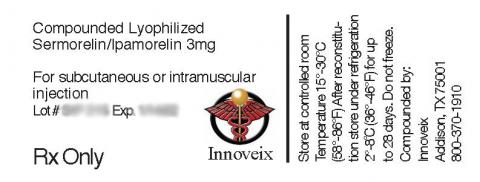
Product DescriptionInjectable Semorelin / Ipamorelin 3mg and injectable AOD-9604 3mg
Company Announcement
Innoveix Pharmaceuticals, Inc. is voluntarily recalling the following lots of sterile compounded drug products, within expiry. The products are being recalled due to a lack of assurance of sterility. These concerns arose following a routine inspection of the pharmacy by FDA.
Administration of a drug product intended to be sterile, that is not sterile, could result in serious infections which may be life-threatening. To date, Innoveix Pharmaceuticals, Inc. has not received any reports of adverse events related to this recall. This voluntary recall is being conducted out of an abundance of caution and to promote patient safety, which is the pharmacy's highest priority.
The affected products are injectable Semorelin / Ipamorelin 3mg and injectable AOD-9604 3mg. The products can be used for various indications as prescribed. The products can be identified by an Innoveix Pharmaceuticals, Inc. label. The products were distributed in glass vials contained in a small 3 inch by 3 inch white box. Products were distributed nationwide to both customers and/or medical facilities. A full list of the affected products with the applicable lot numbers and expiration dates is as follows:
|
Product |
Lot/Expiry |
|---|---|
| Semorelin / Ipamorelin 3mg | Lot# SIP210; Exp: 12/15/2021 Lot# SIP215; Exp: 01/14/2022 Lot# SIP220; Exp: 01/23/2022 |
| AOD-9604 3mg | Lot# AOD205; Exp: 11/09/2021 Lot# AOD210; Exp: 11/18/2021 Lot# AOD 215; Exp: 12/15/2021 Lot# AOD202; Exp: 11/09/2021 |
The pharmacy has notified potentially affected customers of the voluntary recall via U.S. Mail and direct outreach. Customers who have received sterile compounded products subject to the voluntary recall should not use them and return the product to the pharmacy for a full refund.
Consumers with questions regarding this recall can contact Innoveix Pharmaceuticals, Inc. by phone 800-370-1910 or innoveix@gmail.com. We are available Monday through Friday from 9 AM to 4:30 PM CST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using these drug products.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Innoveix
- 800-370-1910
- innoveix@gmail.com
- Media:
- Kevin Hogan
- 214-390-3371