COMPANY ANNOUNCEMENT
Global Pharma Healthcare Issues Voluntary Nationwide Recall of Delsam Pharma Artificial Eye Ointment Due to Possible Microbial Contamination
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionDue to possible microbial contamination
- Company Name:
- Global Pharma Healthcare
- Brand Name:
-
Brand Name(s)Delsam Pharma’s
- Product Description:
-
Product DescriptionArtificial Eye Ointment
Company Announcement
FOR IMMEDIATE RELEASE - February 24, 2023, Global Pharma Healthcare is voluntarily recalling Batch No. H29 of Artificial Eye Ointment, distributed by Delsam Pharma to the consumer level, due to possible microbial contamination. Additionally, some product packaging is leaking or may otherwise be compromised.
Risk Statement: Use of contaminated eye ointment may cause adverse events, including infection in the eye that could lead to blindness. To date, Global Pharma Healthcare has not received any reports of adverse events related to this product.
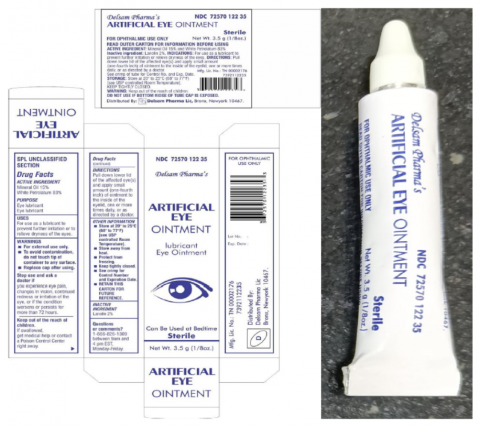
Artificial Eye Ointment (mineral oil 15%, white petrolatum 83%, 3.5 grams / 1/8 oz.) is used as an eye lubricant and to relieve dryness of the eyes. The affected product is packaged in a white aluminum tube within a paper carton. The product can be identified by the photos provided below. The product was distributed nationwide in the United States, and by Delsam through internet retail sites. Delsam Pharma’s NDC for this product is 72570-122-35, and its UPC code is 3 72570 12235 3.
Global Pharma Healthcare is notifying the brand owner and importer of this product, Delsam Pharma, about this recall, and is requesting that wholesalers, retailers, and customers who have the recalled product should stop any use and discard the product safely and appropriately.
Consumers with questions regarding this recall can contact the distributor Delsam Pharma, LLC by phone at 1-866-826-1306 or by e-mail at delsampharma@yahoo.com from Monday to Friday from 11am to 4pm EST.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these over-the-counter drug products.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- distributor Delsam Pharma, LLC
- 1-866-826-1306
- delsampharma@yahoo.com