COMPANY ANNOUNCEMENT
Efficient Laboratories, Inc. Issues Voluntary Nationwide Recall of Rompe Pecho EX, Rompe Pecho CF, and Rompe Pecho MAX due to Microbial Contamination
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason Descriptiondue to Microbial Contamination
- Company Name:
- Efficient Laboratories, Inc.
- Brand Name:
-
Brand Name(s)Efficient
- Product Description:
-
Product DescriptionRompe Pecho EX, Rompe Pecho CF, and Rompe Pecho MAX liquid
Company Announcement
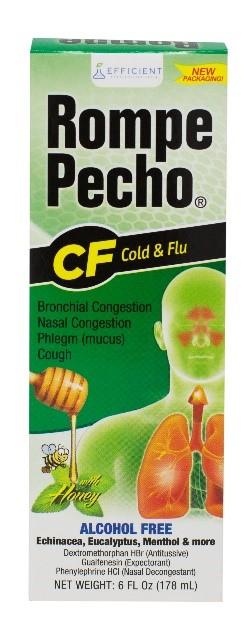
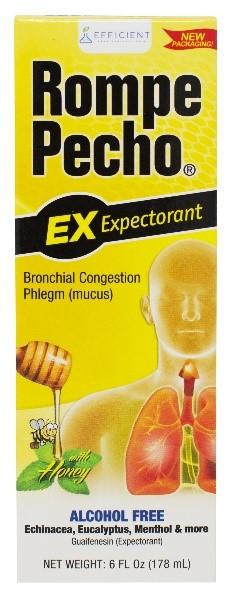
Efficient Laboratories, Inc. is voluntarily recalling one lot each of Rompe Pecho EX, Rompe Pecho CF, and Rompe Pecho MAXliquid. Specifically, we are recalling the following lots: Rompe Pecho EX lot 19F332, exp June 2022, Rompe Pecho CF lot 19H359, exp August 2022 and Rompe Pecho MAX lot 19B42, exp February 2022. These three lots have been found to containmicrobial contamination.
In rare circumstances, consumption of Rompe Pecho from these lots could result in vomiting and diarrhea. Efficient Laboratories has not received any reports of adverse events to date.
These products are used to treat symptoms of the flu and the common cold, and each are packaged in a box containing a bottle of the liquid product. The affected Rompe Pecho product lots are: Rompe Pecho EX lot 19F332, exp June 2022, Rompe Pecho CF lot 19H359, exp August 2022 and Rompe Pecho MAX lot 19B42, exp February 2022. The lot numbers and expiration dates can be found on the bottom of the cartons. These Rompe Pecho products were distributed nationwide to wholesalers and retailers.
Efficient Laboratories is notifying its distributors of these three lots by email and is arranging for the return or replacement of all recalled products.Consumers that have Rompe Pecho EX, Rompe Pecho CF, or Rompe Pecho MAX from these lots thatare being recalled should stop using these productsand discard or return them to the place of purchase.
Consumers with questions regarding this recall can contact Efficient Laboratories by phone at (305) 805-3456, Monday through Friday from 9am to 4:30pm EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Efficient Laboratories
- (305) 805-3456
- Media:
- Melisa Chantres
- (877) 261-6222
- mmchantres@evclay.com