COMPANY ANNOUNCEMENT
Dr. Berne’s Whole Health Products Issues Voluntary Nationwide Recall of Dr. Berne’s MSM Drops 5% and 15% Solution Eye Drops Due to Bacterial and Fungal Contamination
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionBacterial and Fungal Contamination
- Company Name:
- Dr. Berne’s Whole Health Products
- Brand Name:
-
Brand Name(s)Dr. Berne’s
- Product Description:
-
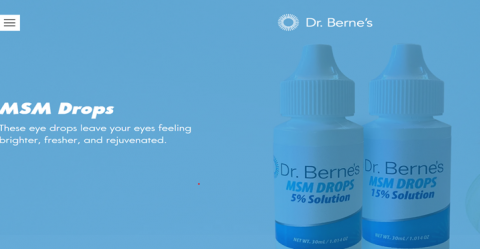
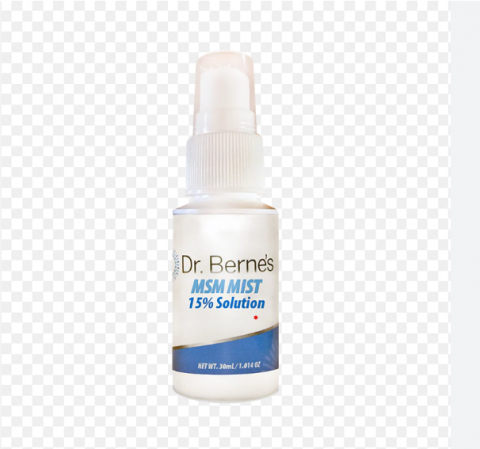
Product DescriptionMSM 5% Solution Eye Drops, MSM 15% Solution Eye Drops, Castor Oil Eye Drops; MSM MIST Drops 5% Solution
Company Announcement
Dr. Berne’s Whole Health Products is voluntarily recalling all lots of MSM DROPS 5%,15% Solution, Dr. Berne’s Organic Castor Oil Eye Drops and Dr. Berne’s MSM MIST 15% Solution to the consumer level. FDA analysis has found one lot (lot 6786) of Dr. Berne's MSM DROPS 5% Solution to fail sterility with both bacterial and fungal contamination found in the product. Out of an abundance of caution, Dr. Berne's is recalling all other lots of the 5% and 15% strengths of MSM Solution and all lots of Dr. Berne’s Organic Castor Oil Eye Drops and Dr. Berne’s MSM MIST 15% Solution.
Risk Statement: Using contaminated eye drops could result in minor to serious vision-threatening infection which could possibly progress to a life-threatening infection. To date, Dr. Berne’s has received 2 reports of adverse events related to this recall.
These products are used as a lubricating eye drop and is packaged in 30 ml/1.014 oz. plastic bottles. The product Dr. Berne’s Organic Castor Oil Eye Drops are packaged in 30 mL/1fl oz white plastic bottle; Dr. Berne’s MSM MIST 15% Solution is packaged in 30mL/1.014 oz white bottle. All these affected products were distributed through Dr. Berne’s webstore.
Dr. Berne’s is notifying its distributors and customers by e-mail and arranging for return of its MSM DROPS 5% and 15% Solution and Dr. Berne’s Organic Castor Oil Eye Drops and Dr. Berne’s MSM MIST 15% Solution. Consumers/distributors/retailers that have product which is being recalled should stop using and return to Sun Star Organics, 988 Main Street, Orange, CA 92867.
Consumers with questions regarding this recall can contact Dr. Berne’s Whole Health Products at (877) 239-3777 or by e-mail at hello@drsamberne.com Mon-Fri 9-5 pm Mountain time Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.