COMPANY ANNOUNCEMENT
Dibar Nutricional S. de R.L. De C.V. Issues Voluntary Nationwide Recall of DIBAR Labs Hand Sanitizer Due to the Presence of Methanol (Wood Alcohol)
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionPresence of methanol
- Company Name:
- Dibar Nutricional S. de R.L. de C.V.
- Brand Name:
-
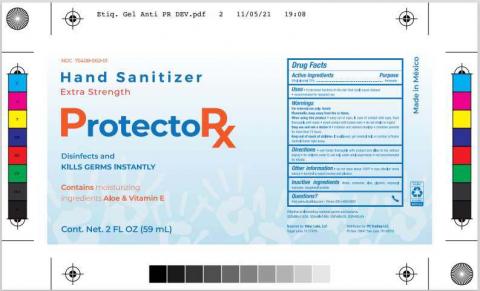
Brand Name(s)DIBAR Labs, ProtectoRx
- Product Description:
-
Product DescriptionHand Sanitizer
Company Announcement
Morelia, Michoacán, Mexico, Dibar Nutricional S. de R.L. De C.V. (“Dibar”) is voluntarily recalling 27 lots of DIBAR Labs Hand Sanitizer packaged in 8oz (8-53090-00301-3 UPC) and 16 oz (8-53090-00302-0 UPC) fluid ounce bottles, respectively, to the consumer level. (See products listed below in Table 1 and Label 1). Dibar is also voluntarily recalling 2 lots of ProtectoRx Hand Sanitizer packaged in 2oz (Lot Number LDHSN050720) and 16oz (Lot Number LDHSN050820) fluid ounce bottles, respectively, to the consumer level. (See products listed below in Table 2 and Label 2). These products are being recalled after testing conducted by our firm revealed the presence of methanol.
Risk Statement: Substantial methanol exposure can result in nausea, vomiting, headache, blurred vision, permanent blindness, seizures, coma, permanent damage to the nervous system, or death. Although all persons using these products on their hands are at risk, young children who accidentally ingest the products and adolescents and adults who drink the products as an alcohol (ethanol) substitute are most at risk for methanol poisoning. To date, the company has not received any reports of adverse reactions related to this recall.
These products are used as hand sanitizers and marketed to help decrease bacteria on the skin when soap and water are not available. The affected bottles of hand sanitizer include a twelvedigit lot code printed on the bottle near the base. The products can be identified by the label, scent, and lot code provided in the table at the end of this release. These products were distributed nationwide in the USA through S.E.N.D. LLC and its customers (Table 1). Products labeled as ProtectoRx Hand Sanitizer were distributed in Puerto Rico through PR TRADING LLC and its customers (Table 2.).
Dibar Nutricional S. de R.L. De C.V., has notified its direct distributors by a letter with confirmatory email and asked that they remove the recalled products from commerce immediately if they still had any in inventory. Consumers, distributors and retailers that have the hand sanitizers which are being recalled should stop using, distributing and/or selling them and return them to the place of purchase.
Consumers with questions regarding this recall can contact our Commercial Offices, +52 443-314-5369, M-F, during business hours 9:00 a.m. – 6:00 p.m. ET and/or email us to QA@dibarlabs.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
(Table 1. Lots distributed to S.E.N.D. LLC and its customers)
|
LOT |
PRODUCT SIZE |
MM/DD/YYYY |
BEST BY |
|---|---|---|---|
| LDHSN041020 | 8 fl oz - 16 fl oz | 04.20.2020 | BEST_BY 04/22 |
| LDHSN041520 | 8 fl oz - 16 fl oz | 04.20.2020 | BEST_BY 04/22 |
| LDHSN041620 | 8 fl oz - 16 fl oz | 04.20.2020 | BEST_BY 04/22 |
| LDHSN041720 | 8 fl oz - 16 fl oz | 04.18.2020 | BEST_BY 04/22 |
| LDHSN042320 | 8 fl oz - 16 fl oz | 04.27.2020 | BEST_BY 04/22 |
| LDHSN042120 | 8 fl oz - 16 fl oz | 04.23.2020 | BEST_BY 04/22 |
| LDHSN042220 | 8 fl oz - 16 fl oz | 04.27.2020 | BEST_BY 04/22 |
| LDHSN042720 | 8 fl oz - 16 fl oz | 05.01.2020 | BEST_BY 04/22 |
| LDHSN043020 | 8 fl oz - 16 fl oz | 05.01.2020 | BEST_BY 04/22 |
| LDHSN042020 | 16 fl oz | 04.23.2020 | BEST_BY 04/22 |
| LDHSN042520 | 16 fl oz | 04.30.2020 | BEST_BY 04/22 |
| LDHSN042420 | 8 fl oz | 04.30.2020 | BEST_BY 04/22 |
| LDHSN042820 | 8 fl oz | 05.01.2020 | BEST_BY 04/22 |
| LDHSN050120 | 8 fl oz - 16 fl oz | 05.04.2020 | BEST_BY 05/22 |
| LDHSN050220 | 16 fl oz | 05.04.2020 | BEST_BY 05/22 |
| LDHSN050420 | 16 fl oz | 05.08.2020 | BEST_BY 05/22 |
| LDHSN050520 | 8 fl oz - 16 fl oz | 05.08.2020 | BEST_BY 05/22 |
| LDHSN050920 | 16 fl oz | 05.08.2020 | BEST_BY 05/22 |
| LDHSN051020 | 8 fl oz | 05.15.2020 | BEST_BY 05/22 |
| LDHSN051120 | 8 fl oz | 05.15.2020 | BEST_BY 05/22 |
| LDHSN051220 | 8 fl oz - 16 fl oz | 05.15.2020 | BEST_BY 05/22 |
| LDHSN052820 | 8 fl oz | 05.29.2020 | BEST_BY 05/22 |
| LDHSN052920 | 8 fl oz | 05.29.2020 | BEST_BY 05/22 |
| LDHSN041520DESC | 8 fl oz | 04.20.2020 | BEST_BY 04/22 |
| LDHSN041620DESC | 8 fl oz | 04.20.2020 | BEST_BY 04/22 |
| LDHSN041720DESC | 8 fl oz | 04.18.2020 | BEST_BY 04/22 |
| LDHSN042120DESC | 8 fl oz | 04.23.2020 | BEST_BY 04/22 |
(Table 2. Lots distributed in Puerto Rico through PR TRADING LLC)
|
LOT |
PRODUCT SIZE |
MM/DD/YYYY |
BEST BY |
|---|---|---|---|
| LDHSN050720 | 2 fl oz | 05.07.2020 | BEST_BY 05/22 |
| LDHSN050820 | 16 fl oz | 05.08.2020 |
BEST_BY 05/22 |
Link to Expanded Recall
Company Contact Information
- Consumers:
- Commercial Offices, José Antonio Díaz Barriga Ahued +52
- 443-314-5369
- QA@dibarlabs.com