COMPANY ANNOUNCEMENT
Dash Xclusive Issues Voluntary Nationwide Recall of Imperia Elita Vitaccino Coffee Due to the Presence of Undeclared Sibutramine and Fluoxetine
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionUndeclared Sibutramine and Fluoxetine
- Company Name:
- Dash Xclusive
- Brand Name:
-
Brand Name(s)Imperia Elita
- Product Description:
-
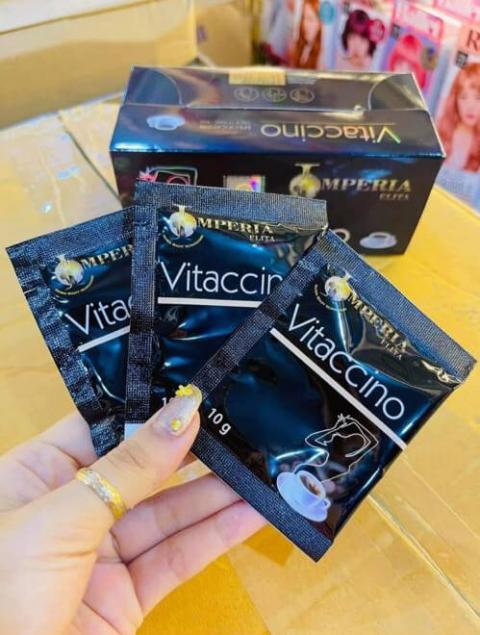
Product DescriptionImperia Elita Vitaccino Coffee
Company Announcement
Dash Xclusive is voluntarily recalling all lots of Imperia Elita Vitaccino Coffee to the consumer level. FDA analysis has found the product to contain undeclared sibutramine and fluoxetine.
Sibutramine was an FDA-approved drug used as an appetite suppressant for weight loss but was withdrawn from the market because of safety issues, including stroke, heart failure and serious health risk especially to those with underlying heart related disease. Fluoxetine is an FDA approved drug indicated for the treatment of various depressive disorders, obsessive compulsive disorder, bulimia and panic disorders. This drug product carries a box warning for suicidal thoughts and behaviors and it needs to be monitored closely by a prescriber. The presence of sibutramine and fluoxetine in Imperia Elita Vitaccino coffee renders it an unapproved drug for which safety and efficacy has not been established and therefore subject to a recall. Dash Xclusive has not received any reports of adverse events related to this recall.
Risk Statement: Products containing undeclared sibutramine pose a threat to consumers because sibutramine is known to substantially increase blood pressure and/or pulse rate in some patients and may present a significant risk for patients with a history of coronary artery disease, congestive heart failure, arrhythmias or stroke. Products containing undeclared fluoxetine could cause suicidal thinking and behavior, an accumulation of high levels of serotonin in the body or neuroleptic malignant syndrome (NMS)-like reactions which is a life-threatening neurological disorder; elevated mood state that can cause higher than normal energy levels, restlessness, decreased need for sleep; abnormal bleeding, and low sodium levels.
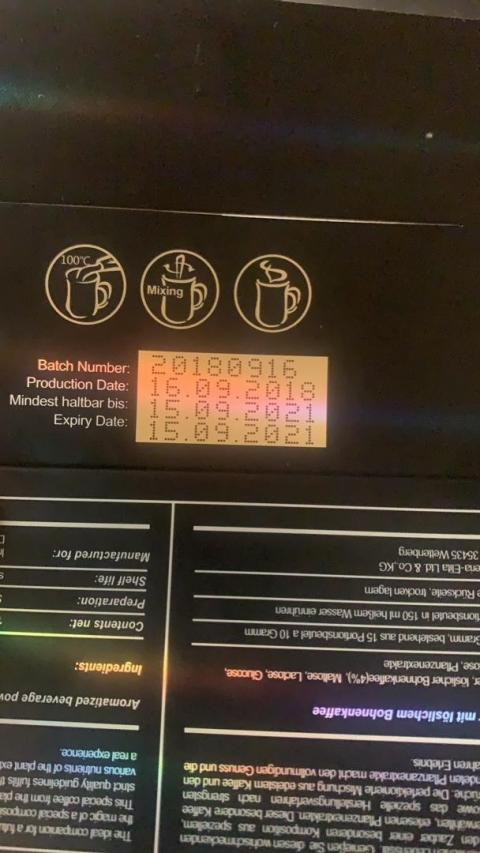
The product is marketed as a dietary supplement for weight loss and is packaged in a black rectangular box and contains fifteen sachets. The affected Imperia Elita Vitaccino coffee lots include all lots. Product was distributed Nationwide in the USA via internet and by ebay at www.ebay.com. On December 17, 2020, FDA issued a press release that warned consumers to avoid certain products found on Amazon, eBay and other retailers due to hidden and potentially dangerous drug ingredients. It also encouraged online marketplaces to ensure these products are not sold on their platforms.
Dash Xclusive is notifying its customers by e-messages on the eBay platform and is arranging for return of all recalled products. Consumers that have Imperia Elita Vitaccino Coffee which is being recalled should stop using/return to Dash Xclusive, PO BOX. #220, 1125 E. Broadway Glendale, California 91205.
Consumers with questions regarding this recall can contact Dash Xclusive by e-mail at dashxclusive11@gmail.com on Mondays to Thursdays from 11am to 4pm Pacific Time zone. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.