COMPANY ANNOUNCEMENT
Blaine Labs Issues Voluntary Nationwide Recall of RevitaDerm Wound Care Gel Due to Bacterial Contamination
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionMay be contaminated with Bacillus cereus
- Company Name:
- Blaine Labs Company
- Brand Name:
-
Brand Name(s)RevitaDerm
- Product Description:
-
Product DescriptionWound Care Gel
Company Announcement
SANTA FE SPRINGS, Calif., Jan. 27, 2022 -- Blaine Labs Company is voluntarily recalling one lot of RevitaDerm Wound Care to the consumer level because a bottle of the 1.0 ounce RevitaDerm Wound Care Gel has been found to be contaminated with Bacillus cereus.
Risk Statement: Patients who apply the contaminated product to a wound could develop a skin and soft tissue infection which could lead to serious complications. For non-immunocompromised patients these infections are expected to be less severe and responsive to treatment. However, for the immunocompromised patients and preterm neonates, Bacillus cereus can cause life-threatening, invasive infections including wound and blood infections, sepsis, pneumonia, and meningitis. To date, Blaine Labs Company has not received any complaints or reports of adverse events related to this lot of 1.0 ounce bottle or 3.0 ounce tube of RevitaDerm Wound Care Gel.
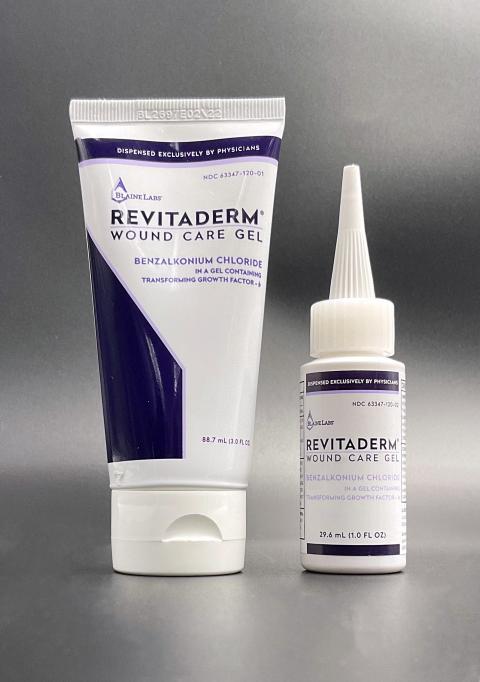
The product is used as a skin wound antimicrobial and is packaged in a 1.0 ounce bottle and a 3.0 ounce tube with each labeled as "RevitaDerm Wound Care Gel" with a Drug Facts label on the back. The affected RevitaDerm Wound Care Gel lot is BL 2844 with an expiration date of 02/19/2023. The product can be identified by its name on the front of either the 1.0 ounce bottle or the 3.0 ounce tube, and the 1.0 ounce volume has the witch-hat dispensing cap. The RevitaDerm Wound Care Gel Product was distributed to 61 physician clinics in 17 states in the United States in the year 2021.
Blaine Labs is notifying its physician clients by email, regular mail and by phone, and is arranging for the return of undispensed 1.0 ounce bottles and 3.0 ounce tubes from lot BL 2844. Patients who have the RevitaDerm Wound Care Gel 1.0 ounce bottle or 3.0 ounce tubes, which are being recalled, should stop using the product and return unused product to the dispensing physician.
Consumers with questions regarding this recall can contact Blaine Labs at 800-307-8818 or email info@blainelabs.com Monday through Friday from 8:00AM to 4:00PM PST. Consumers should contact their physicians or healthcare provider if they have experienced any problems that may be related to using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact:
Paul Kubin
800-307-8818