Lung Injuries Associated with Use of Vaping Products
Information for the Public, FDA Actions, and Recommendations

Background
Both the U.S. Food and Drug Administration and the U.S. Centers for Disease Control and Prevention are working tirelessly to investigate the distressing incidents of severe respiratory illness associated with use of vaping products. The FDA and CDC are working closely with state and local health officials to investigate these incidents as quickly as possible, and we are committed to taking appropriate actions as a clearer picture of the facts emerges.
FDA Statement on consumer warning to stop using THC vaping products amid ongoing investigation into lung illnesses - October 4, 2019
While the work by federal and state health officials to identify more information about the products used, where they were obtained and what substances they contain is ongoing, the FDA is providing consumers with some information to help protect themselves.
- Incident Overview
- FDA Actions
- FDA Preliminary Lab Analysis
- Information & Resources for Consumers
- Information for Healthcare Providers
- Information for State Health Departments
- FDA Resources
- CDC Resources
Incident Overview
- The Centers for Disease Control and Prevention (CDC) is working with states to determine if cases are confirmed or probable after examining the medical records of suspected cases.
- Please see the CDC’s website for updated cases. These numbers may change frequently.
- While some cases in each of the states are similar and appear to be linked to vaping product use, more information is needed to determine what is causing the respiratory illnesses.
- In many cases, patients reported a gradual start of symptoms, including breathing difficulty, shortness of breath, and/or chest pain before hospitalization. Some cases reported mild to moderate gastrointestinal illness including vomiting and diarrhea, or other symptoms such as fevers or fatigue.
- In many cases, patients told healthcare personnel or health department staff of recent use of vaping products containing tetrahydrocannabinol (THC, a psychoactive component of the marijuana plant).
- Even though cases appear similar, it is not clear if they have a common cause or if they involve different diseases with similar presentations, which is an issue central to our investigation.
- CDC and the FDA are providing consultation to state health departments and are working closely with them to gather information on any products or substances used.
- For example, our agencies are working to standardize information collection at the state level to help build a more comprehensive picture of these incidents. This includes investigating the brand, manufacturer and types of vaping products, whether any of them are products that would fall within the FDA’s regulatory authority, as well as where they were obtained.
FDA Actions
Former Acting FDA Commissioner Ned Sharpless, M.D. takes a tour of the FDA’s Forensic Chemistry Center. The Center serves as the FDA’s premier national laboratory and is playing a critical role in fact-gathering and analysis for the ongoing incidents of lung illnesses following vaping product use.
- The FDA remains deeply concerned about these respiratory illnesses and deaths and is working closely with the CDC, as well as state and local public health partners, to investigate them as quickly as possible.
- To help gather and analyze as much information as possible, the FDA’s laboratory is working closely with our federal and state partners to identify the products or substances that may be causing the illnesses.
- The FDA is analyzing samples submitted by a number of states for the presence of a broad range of chemicals, including nicotine, THC and other cannabinoids, along with cutting agents/diluents and other additives, pesticides, opioids, poisons, heavy metals and toxins.
- No one substance has been identified in all of the samples tested. Importantly, identifying any compounds that are present in the samples will be one piece of the puzzle but will not necessarily answer questions about what is causing these illnesses.
- Federal and state partners are following any potential leads, and the FDA is committed to taking appropriate actions as the facts emerge and keeping the public informed as we have more information to share.
FDA Preliminary Lab Analysis
- To date, the FDA has received over 1,300 samples from 31 states and one territory with roughly 1,090 of these samples connected to patients. These samples have been collected directly from consumers, hospitals, and state offices. They have included vaping devices and products containing varied levels of liquid as well as packaging and other documentation. Many samples have contained little to no liquid, which limits the number and types of tests that can be conducted on each submission. The FDA has not found one product or substance that is involved in all of the cases; however, we do know that THC is present in most of the samples being tested. The following is a snapshot of lab activities most relevant to the samples containing THC, as of the date noted.
- As of Feb. 12, 2020, 843 of the 1,090 samples connected to patients (77%) have undergone some level of testing and additional testing is likely to be conducted on many of these products.
- 511 of these samples have been found to contain THC
- 50% of the THC products have been found to contain vitamin E acetate as a diluent. The concentration of vitamin E acetate determined in a subset of these samples has ranged from 23% to 88%
- 29% of the THC products have been found to contain another diluent such as medium chain triglycerides
- 511 of these samples have been found to contain THC
- The FDA labs are also doing work to focus on connecting the analysis of samples to particular patients with assigned CDC case numbers. As of Feb 12, 2020, approximately 677 samples are directly linked to 95 patients with CDC case numbers and samples from 93 of these patients have been analyzed.
- 73% of these 93 patients were connected to products containing THC.
- Of these:
- 81% of cases included products with vitamin E acetate as a diluent
- 32% included products with aliphatic esters as diluent (e.g., triglycerides)
- 9% included products with polyethylene glycol as diluent
- Of these:
- 73% of these 93 patients were connected to products containing THC.
- It is important to stress that identifying any compounds present in the samples linked to patient cases is but one piece of the puzzle and will not necessarily answer questions about causality, which makes ongoing work critical at both the state and federal levels. Every day the FDA and partners are gathering more information and seek to use that information to better understand the relationship between any specific products or substances and the reported illnesses. Importantly, the variations of use patterns, products or substances reportedly used and the samples being tested may mean there are multiple causes of these injuries.
Information & Resources for Consumers
- CDC, FDA, and state health authorities have made progress in identifying substances of concern in e-cigarette, or vaping, product use-associated lung injury (EVALI), and in characterizing the outbreak.
- National emergency department data and active case reporting from state health departments around the country show a sharp rise in symptoms or cases of EVALI in June 2019, a peak in September 2019, and a gradual, but persistent decline since then.
- Although cases related to the outbreak are decreasing, new cases continue to be reported to CDC by state health departments and samples connected to EVALI patients continue to be tested by both CDC and FDA.
- National and state data from patient reports and product sample testing suggest THC-containing e-cigarette, or vaping, products, particularly from informal sources like friends, or family, or in-person or online dealers, are linked to most EVALI cases and play a major role in the outbreak.
- Vitamin E acetate is strongly linked to the EVALI outbreak. Vitamin E acetate has been found in product samples tested by FDA and state laboratory and in patient lung fluid samples tested by CDC from geographically diverse states. Vitamin E acetate has not been found in the lung fluid of people that do not have EVALI.
- However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC or non-THC products, in some of the reported EVALI cases.
- FDA and CDC recommend that people not use THC-containing e-cigarette, or vaping, products, particularly from informal sources like friends, or family, or in-person or online dealers.
- Vitamin E acetate should not be added to any e-cigarette, or vaping, products. Additionally, people should not add any other substances not intended by the manufacturer to products, including products purchased through retail establishments.
- Adults using nicotine-containing e-cigarette or vaping products as an alternative to cigarettes should not go back to smoking; they should weigh all available information and consider using FDA-approved smoking cessation medications. They should contact their healthcare professional if they need help quitting tobacco products, including e-cigarettes, as well as if they have concerns about EVALI.
- E-cigarette, or vaping, products should never be used by youths, young adults, or women who are pregnant. Adults who do not currently use tobacco products should not start using e-cigarette, or vaping, products.
- THC use has been associated with a wide range of health effects, particularly with prolonged frequent use. The best way to avoid potentially harmful effects is to not use THC-containing e-cigarette, or vaping, products.
- Persons engaging in ongoing cannabis use that leads to significant impairment or distress should seek evidence-based treatment by a health care professional.
- If you are concerned about your health after using a vaping product, contact your health care provider, or you can also call your local poison control center at 1-800-222-1222.
- If you experience vaping-associated respiratory illness, the FDA also encourages you to report this information, including providing any associated product, to your state or local health departments. Reporting to your state or health departments is crucial as federal and state partners work together to have accurate case identification and reported case counts.
- If you experience a problem with any tobacco product, such as an unexpected health or safety issue, report it online using the Safety Reporting Portal. You may submit reports about any tobacco product, including cigarettes, roll-your-own cigarettes, cigars, smokeless tobacco, electronic cigarettes and waterpipe tobacco. You can also report problems with the components and parts of tobacco products. The FDA website has more information on what to include in a report.
Information for Healthcare Providers
- As this investigation continues, CDC and the FDA encourage clinicians to report possible cases of vaping-associated respiratory illness to their local or state health department for further investigation. Reporting to your state or health departments is crucial as federal and state partners work together to have accurate case identification and reported case counts.
- If vaping product use is suspected as a possible cause for a patient’s lung illness, a detailed history of the substances used, the sources, and the devices used should be obtained, as outlined in the CDC Health Advisory, and efforts should be made to determine if any remaining product, devices, and liquids are available for testing. Health care providers also can contact their local poison control center.
- Product-specific information related to possible cases may be submitted to the FDA online using the Safety Reporting Portal. The FDA website also has more information on what to include in a report to the SRP.
Information for State Health Departments
- FDA would like to thank all State Departments of Health (and other partners) for their ongoing efforts, collaboration and communications on the recent respiratory illnesses associated with e-cigarettes and vaping products.
- CDC and FDA are working together to coordinate analysis of e-cigarette, or vaping, products to provide insight into the nature of the chemical exposure(s) contributing to the lung injury outbreak.
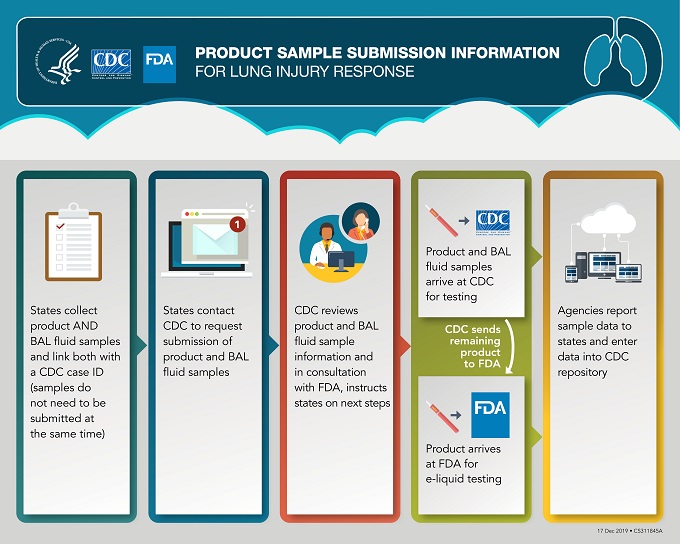
- As of Dec. 19, 2019, CDC and FDA are accepting case-associated product samples for aerosol or e-liquid testing if corresponding bronchoalveolar lavage (BAL) fluid samples are submitted to CDC (samples do not need to be submitted at the same time to meet this requirement). CDC and FDA will no longer be accepting isolated case-associated product samples.
- In EVALI-related death cases, CDC and FDA will continue to accept product samples for testing even if no BAL fluid sample is available for testing.
- This change does not mean that the investigation has concluded. The extensive product testing conducted by both FDA and CDC to date has provided critical information regarding potential chemicals of concern. At this time, additional focused testing is needed for paired specimen testing (i.e., both product and BAL fluid samples) in order to continue to further inform the investigation.
- Additionally, CDC and FDA are streamlining the sample collection process to reduce the burden on state partners. All product samples and corresponding BAL fluid samples will be submitted to CDC. CDC and FDA will coordinate sample testing and transfer to testing laboratories as appropriate.
- CDC will conduct aerosol emissions testing of e-cigarette, or vaping, products. FDA is analyzing e-liquids for the presence of a broad range of chemicals. Analysis of both aerosol emissions and e-liquids will complement each other, and together will help improve our understanding of exposures among case patients associated with the lung injury outbreak.
- The graphic below outlines the process for states to submit product samples to CDC for routing and testing in coordination with FDA. Upon receipt of product samples, CDC will determine whether sufficient liquid product is available to perform aerosol and e-liquid analysis and coordinate testing with FDA.
- For information about collection and submission of e-cigarette or other vaping products, including e-liquids, associated with confirmed or probable cases for possible testing by FDA, contact: FDAVapingSampleInquiries@fda.hhs.gov.
- For information about collection and submission of e-cigarette or other vaping products’ e-liquids associated with confirmed or probable cases for possible aerosol emissions testing by CDC, contact IncidentResponse@cdc.gov.
FDA Resources
- FDA Information on Vaporizers, E-Cigarettes, and other Electronic Nicotine Delivery Systems (ENDS)
- FDA Sample Collection Criteria and Information for Vaping Related Incidents
- FDA Safety Reporting Portal
- Information to Include in Tobacco Product Problem Report
- FDA Lung Injury Update: FDA Warns Public to Stop Using Tetrahydrocannabinol (THC)-Containing Vaping Products and Any Vaping Products Obtained Off the Street
CDC Resources
- CDC Outbreak Information
- CDC Health Alert on Pulmonary Disease Associated with Using Vaping Products
- Transcript of Telebriefing: Update on Lung Injury Associated with E-cigarette Use, or Vaping – Oct. 11, 2019
- Transcript of Telebriefing: Lung Injury Investigation – Oct. 3, 2019
- Transcript of Telebriefing: CDC Update on Pulmonary Illnesses – Sept. 27, 2019
- Transcript of Telebriefing: Update on Lung Injury Associated with E-cigarette Product Use, or Vaping - Sept. 19, 2019
- Transcript of Telebriefing: Investigation of Pulmonary Disease Among People Who Use E-cigarettes - Sept. 6, 2019
- Transcript of Telebriefing: Severe Pulmonary Disease Associated with Use of E-cigarettes - Aug. 23, 2019