Medical Gowns
Medical gowns are examples of personal protective equipment used in health care settings. They are used to protect the wearer from the spread of disease-causing microorganisms if the wearer comes in contact with potentially infectious liquid or solid material. They may also be used to help prevent the wearer from transferring microorganisms that could harm vulnerable patients, such as those with compromised immune systems. Gowns are intended to provide broad barrier protection. At this time, the FDA has not cleared, approved, or authorized any gowns for specific protection or prevention against the virus that causes COVID-19. Gowns are one part of an overall infection-control strategy.
Many names are used to refer to gowns intended for use in health care settings, including, surgical gowns, isolation gowns, surgical isolation gowns, non-surgical gowns, procedural gowns, and operating room gowns.
On this page:
- Levels of Medical Gowns
- Surgical Gowns
- Surgical Isolation Gowns
- Non-Surgical Isolation Gowns
- Standards for Gowns
- Sterility Information for Gowns
- Biocompatibility Information for Gowns
- Choosing Which Gown to Use
Levels of Medical Gowns
When choosing gowns, look for product labeling that describes intended use with the desired level of protection based on the risk levels described below.
The FDA recognizes the consensus standard American National Standards Institute/Association of the Advancement of Medical Instrumentation (ANSI/AAMI) PB70, "Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities." This standard establishes a system of classification for protective apparel and drapes used in healthcare facilities based on their liquid barrier performance and specifies related labeling requirements and standardized test methods for determining compliance. The standard is intended to ultimately assist end-users in determining the type(s) of protective product most appropriate for a particular task or situation:
- Level 1: Minimal risk, to be used, for example, during basic care, standard isolation, cover gown for visitors, or in a standard medical unit
- Level 2: Low risk, to be used, for example, during blood draw, suturing, in the Intensive Care Unit (ICU), or a pathology lab
- Level 3: Moderate risk, to be used, for example, during arterial blood draw, inserting an Intravenous (IV) line, in the Emergency Room, or for trauma cases
- Level 4: High risk, to be used, for example, during long, fluid intense procedures, surgery, when pathogen resistance is needed or infectious diseases are suspected (non-airborne)
Surgical Gowns
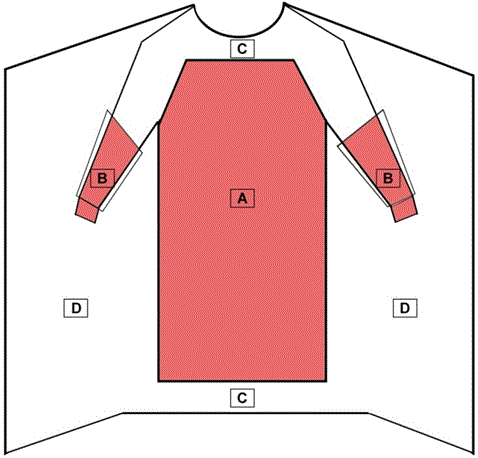
A surgical gown is regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification. A surgical gown is a personal protective garment intended to be worn by health care personnel during surgical procedures to protect both the patient and health care personnel from the transfer of microorganisms, body fluids, and particulate matter. Because of the controlled nature of surgical procedures, critical zones of protection have been described by national standards. As referenced in Figure 1: the critical zones include the front of the body from top of shoulders to knees and the arms from the wrist cuff to above the elbow. All surgical gowns must be provided sterile and labeled as a surgical gown.
Figure 1 - Critical Zones for Surgical Gowns
- The entire front of the gown (areas A, B, and C) is required to have a barrier performance of at least level 1.
- The critical zone compromises at least areas A and B.
- The back of the surgical gown (area D) may be nonprotective.
Surgical Isolation Gowns
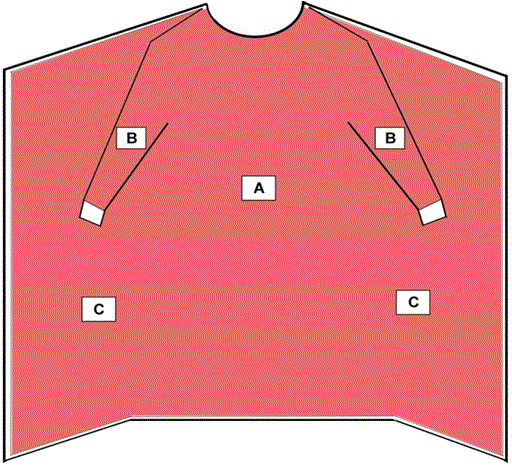
Surgical isolation gowns are used when there is a medium to high risk of contamination and a need for larger critical zones than traditional surgical gowns. Surgical isolation gowns, like surgical gowns, are regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification. As referenced in Figure 2, all areas of the surgical isolation gown except bindings, cuffs, and hems are considered critical zones of protection and must meet the highest liquid barrier protection level for which the gown is rated. All seams must have the same liquid barrier protection as the rest of the gown. Additionally, the fabric of the surgical isolation gown should cover as much of the body as is appropriate for the intended use.
Non-Surgical Isolation Gowns
Non-surgical isolation gowns are Class I devices (exempt from premarket review) intended to protect the wearer from the transfer of microorganisms and body fluids in low or minimal risk patient isolation situations. Non-surgical gowns are not worn during surgical procedures, invasive procedures, or when there is a medium to high risk of contamination.
Like surgical isolation gowns, non-surgical gowns should also cover as much of the body as is appropriate to the task. As referenced in Figure 2, all areas of the non-surgical gown except bindings, cuffs, and hems are considered critical zones of protection and must meet the highest liquid barrier protection level for which the gown is rated. All seams must have the same liquid barrier protection as the rest of the gown.
Figure 2 - Critical Zones for Surgical Isolation Gowns and Non-Surgical Isolation Gowns
- The entire gown (areas A, B, and C), including seams but excluding cuff, hems, and bindings, is required to have a barrier performance of at least Level 1.
- Surgical isolation gowns are used when there is a medium to high risk of contamination and need for larger critical zones than traditional surgical gowns.
Non-sterile, non-isolation gown intended to provide moderate or high barrier protection
Non-sterile, non-isolation gowns are intended to be worn by health care personnel to provide moderate or high barrier protection in non-sterile and nonpatient isolation situations. These gowns regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification.
Non-surgical non-isolation gown
Non-surgical non-isolation gowns are intended to be worn by health care personnel to provide minimal or low barrier protection in non-surgical and non-patient isolation situations. These gowns are Class I devices (exempt from premarket review).
Cloth gowns that will not be used in a sterile field, such as surgery, can be reused if they are laundered in enzymatic detergent or per the hospital's standard operating procedures.
Standards for Gowns
The FDA recognizes consensus standards for gowns as listed in the FDA's Recognized Consensus Standards database.
Sterility Information for Gowns
For a device sold sterile, the FDA recommends sponsors provide the following information as detailed in the final guidance entitled Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile. This information may include:
- Sterilization method that will be used.
- A description of the method that will be used to validate the sterilization cycle, but not the validation data itself (for established sterilization methods).
- Reference to a standard method (e.g., AAMI Radiation Standard) usually is sufficient for established sterilization methods with FDA-recognized standards.
- The sterility assurance level (SAL) for the device which the firm intends to meet. An SAL of 10-6 is required for surgical drapes and surgical gowns which are to be used during surgical procedures.
- A description of the packaging's ability to maintain the device's sterility.
- If sterilization involves ethylene oxide (EtO), the maximum levels of residues of ethylene oxide and ethylene chlorohydrin that remain on the device. The levels should be consistent with the FDA-recognized consensus standards for ethylene oxide.
- In the case of radiation sterilization, the radiation dose.
Biocompatibility Information for Gowns
Medical gowns are considered surface devices, contacting breached or compromised surfaces, with limited contact duration (≤ 24 hours), per FDA’s Guidance on Premarket Notification [510(k)] Submissions for Surgical Gowns and Surgical Drapes. The FDA recommends that cytotoxicity (ISO 10993-5), sensitization (ISO 10993-10), and irritation (ISO 10993-23) endpoints be evaluated. For more information about biocompatibility, see the FDA's Biocompatibility Assessment Resource Center.
Choosing Which Gown to Use
To identify FDA-cleared products, search the 510(k) Premarket Notification database using the product codes for gowns (FYA, FYB, FYC, and QPC). The CDC also provides CDC Guidance for the Selection and use of PPE in Healthcare Settings (PDF file).
Check if the gown has expired, as indicated by the manufacturer-designated shelf life in the product labeling. Expired gowns may be used for training and demonstration purposes where barrier protection is not needed.
Additional Information
- Guidance: Premarket Notification Requirements Concerning Gowns Intended for Use in Health Care Settings - Guidance for Industry and Food and Drug Administration Staff
- Guidance on Premarket Notification [510(k)] Submissions for Surgical Gowns and Surgical Drapes
- Guidance: Use of International Standard ISO 10993-1, "Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process
- Guidance: Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile