Counterfeit At-Home OTC COVID-19 Diagnostic Tests
The FDA is aware of counterfeit at-home over-the-counter (OTC) COVID-19 diagnostic tests being distributed or used in the United States. These counterfeit tests should not be used or distributed.
Counterfeit COVID-19 tests are tests that are not authorized, cleared, or approved by the FDA for distribution or use in the United States, but are made to look like authorized tests so the users will think they are the real, FDA-authorized test. The performance of these counterfeit tests has not been adequately established and the FDA is concerned about the risk of false results when people use these unauthorized tests.
You may risk unknowingly spreading COVID-19 and may delay or stop appropriate medical treatment for COVID-19 if you use a counterfeit test.
- A false-negative antigen test result means that the test says the person does not have COVID-19 but they actually do have COVID-19. A false-negative result may lead to delayed diagnosis or inappropriate treatment of SARS-CoV-2, which may cause people harm including serious illness and death. False-negative results can also lead to further spread of the SARS-CoV-2 virus, including when people are housed together in health care, long-term care, and other facilities based on these false test results. When false negative test results are received, actions to limit exposure to an infected person might not be taken, such as isolating people, limiting contact with family and friends, or limiting access to places of employment.
- A false-positive antigen test result means that the test says the person has COVID-19 but they actually do not have COVID-19. A false-positive result may lead to a delay in both the correct diagnosis and appropriate treatment for the actual cause of a person’s illness, which could be another life-threatening disease that is not COVID-19. False-positive results could also lead to further spread of the SARS-CoV-2 virus when presumed positive people are housed together.
The FDA will update this page to list counterfeit at-home OTC COVID-19 diagnostic tests to alert the public, including test users, caregivers, health care providers, and distributors, and to provide information on how to identify counterfeit tests.
This page provides information on:
- How do I know if my at-home OTC COVID-19 diagnostic test is FDA-authorized?
- What are some signs that an at-home OTC COVID-19 diagnostic test may be counterfeit?
- What products has FDA identified as counterfeit at-home OTC COVID-19 diagnostic tests?
- What should I do if I have a counterfeit at-home OTC COVID-19 diagnostic test?
- What is the FDA doing about counterfeit at-home OTC COVID-19 diagnostic tests?
- How do I report a problem with an at-home OTC COVID-19 diagnostic test?
How do I know if my at-home OTC COVID-19 diagnostic test is FDA-authorized?
The FDA has a list of authorized at-home OTC COVID-19 diagnostic tests. For more information about each test, including the Letter of Authorization and authorized labeling, see In Vitro Diagnostics EUAs: Tables of IVD EUAs.
Check this page regularly to see if the FDA is aware of counterfeit versions of the tests. If the test you have has the same name as one listed on this page, follow the instructions below to check for signs that it is counterfeit or to confirm that it is the real, FDA-authorized product. You can also contact the manufacturer of the test if you have questions or concerns, and they will be able to help you determine if your test is FDA-authorized or counterfeit.
The FDA is not aware of any counterfeit tests distributed by the U.S. Government test distribution programs.
What are some signs that an at-home OTC COVID-19 diagnostic test may be counterfeit?
- Poor print quality of images or text on the outside box label for the product or in the instructions for use included in the box.
- Missing information on the outside box label for the product, such as the lot number, expiration date, or barcode or QR codes.
- Grammatical or spelling errors found in product labeling.
- Components of the kits do not match the content description (for example, missing Instructions for Use, missing or unfilled components, different number of components than listed).
- Tradename for product printed on component or box labels differ from the authorized labeling found on the FDA website: At-Home OTC COVID-19 Diagnostic Tests | FDA.
- The box label or printed instructions for use look different from the authorized labeling found on the FDA website: At-Home OTC COVID-19 Diagnostic Tests | FDA.
What products has the FDA identified as counterfeit at-home OTC COVID-19 diagnostic tests?
Do not use counterfeit Flowflex COVID-19 Test Kits.
The FDA is aware that counterfeit versions of the FDA-authorized Flowflex COVID-19 Antigen Home Tests are being illegally imported and distributed in the United States through unauthorized distributors and resellers who have no connection to ACON Laboratories, Inc. These counterfeit tests have not been reviewed or authorized by FDA, but current evidence suggests the counterfeit tests are not performing as well as the authorized tests. The packaging and components of the fraudulent tests very closely resemble real, FDA-authorized Flowflex tests. The FDA-authorized Flowflex tests are still safe to use when following the authorized instructions for use.
The fraudulent tests discussed on this page are not the same as the previously reported issue with the unauthorized ACON Biotech Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing). In March 2021, the FDA warned people not to use certain ACON Biotech Flowflex COVID-19 tests packaged in a dark blue box because they have not been cleared or approved by the FDA for distribution or use in the United States.
The FDA is providing the information on this page to help consumers identify counterfeit test kits that imitate the FDA-authorized Flowflex COVID-19 Antigen Home Tests (in white boxes), but are not authorized, cleared, or approved by the FDA for distribution or use in the United States. ACON Laboratories, Inc. has also issued a public notice of this issue.
Below are images and descriptions comparing the counterfeit product to the FDA-authorized test.
- The counterfeit white retail boxes are missing the Lot Number / Expiration Date / 2D-datamatrix label that is found on FDA-authorized Flowflex COVID-19 Antigen Home Tests:
- The counterfeit test kits are missing the Spanish language Instructions For Use. The FDA-authorized Flowflex test kits include both English and Spanish Instructions For Use.
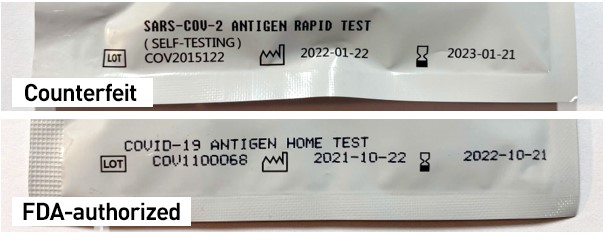
- The counterfeit pouches inside the box that contain the test cassette are labeled as “SARS-COV-2 ANTIGEN RAPID TEST (SELF-TESTING),” whereas the FDA-authorized Flowflex test cassette pouches are labeled “COVID-19 ANTIGEN HOME TEST”:
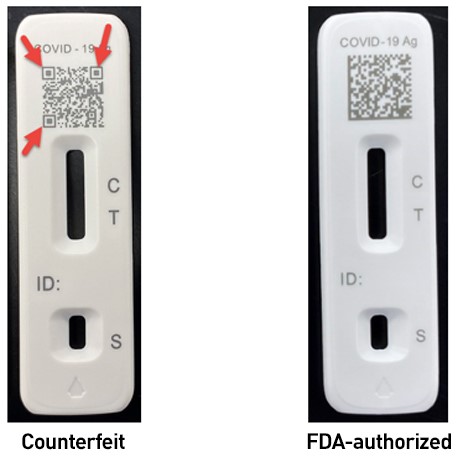
- The plastic test cassette may have a 2D barcode which differs from the one printed on FDA-authorized Flowflex tests. The counterfeit tests may have a QR code containing square shapes within a square box on 3 corners of the code, as shown below. The FDA-authorized Flowflex tests have a 2D-datamatrix without those three boxes.
Do not use counterfeit iHealth COVID-19 Antigen Rapid Test Kits.
The FDA is aware that counterfeit versions of the FDA-authorized iHealth COVID-19 Antigen Home Tests are being illegally imported and distributed in the United States through unauthorized distributors and resellers who have no connection to iHealth Labs, Inc. These counterfeit tests have not been reviewed or authorized by FDA, and the FDA has no current evidence of their performance. The packaging and components very closely resemble real, FDA-authorized iHealth tests. The FDA-authorized iHealth tests are still safe to use when following the authorized instructions for use.
Below are images and descriptions comparing the counterfeit product to the FDA-authorized test.
- The FDA is aware that there are not many easily identifiable differences between the counterfeit tests and authorized iHealth tests. There may also be different versions of the counterfeit tests. The FDA is continuing to work with iHealth on how to best identify the counterfeit tests. The general signs that an OTC COVID-19 diagnostic test may be counterfeit provided above on this page may apply to some of these counterfeit versions (for example: poor print quality of images or text on the outside box label for the product or in the instructions for use included in the box or grammatical or spelling errors found in product labeling.)
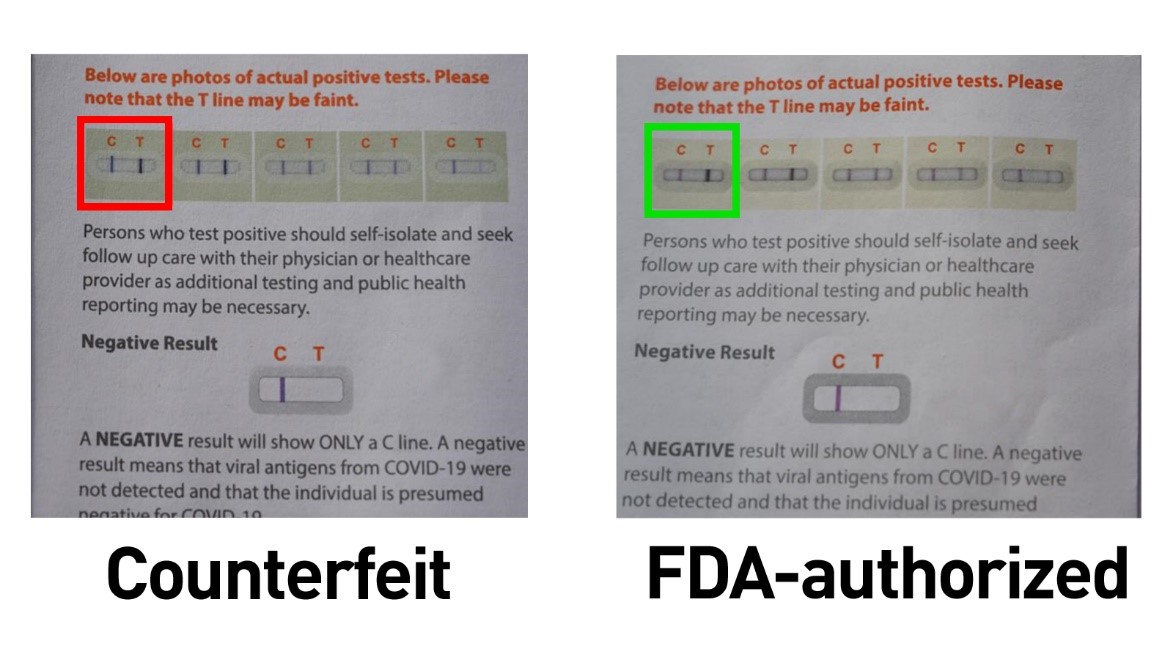
- In some cases, the paper Instructions for Use included in the box with the counterfeit test may have a minor difference in the images showing examples of positive test results. As shown in the red box in the image below, the line is black and extends past the top of the “results window”:
What should I do if I have a counterfeit at-home OTC COVID-19 diagnostic test?
Do not use the counterfeit tests. Contact the distributor or store where you purchased the test to inform them that you received a counterfeit test, and also inform the manufacturer of the authorized test. The manufacturer may ask you for additional information such as photos of the packaging to further investigate the issue. After providing any requested information to the distributor and/or manufacturer, follow the manufacturer’s instructions for returning or disposing of the test.
Talk to your health care provider if you think you were tested with a counterfeit test and you have concerns about your test results.
What is the FDA doing about counterfeit at-home OTC COVID-19 diagnostic tests?
The distribution of counterfeit COVID-19 products is a threat to the public health. The FDA regularly monitors the marketing of unauthorized, unapproved, or uncleared tests, including reports of problems with test performance or results. The FDA is working with manufacturers to address this safety issue.
The FDA will continue to keep the public informed of significant new information.
How do I report a problem with an at-home OTC COVID-19 diagnostic test?
If you think you had a problem with a COVID-19 test, the FDA encourages you to report the problem through the MedWatch Voluntary Reporting Form.
Health care personnel employed by facilities that are subject to the FDA's user facility reporting requirements should follow the reporting procedures established by their facilities.