FY2015 Regulatory Science Research Report: Modified Release Drug Products: Therapeutic Equivalence between Brand-Name Drugs and Generics
Introduction
Among the top 200 prescribed drugs in USA, one third are modified drug products. While modified release drug products provided unique clinical benefits, modified-release (MR) solid oral dosage forms have more failure modes than immediate-release (IR) products.
- Modern controlled release technologies can produce a wide range of differently shaped PK profiles that can meet traditional BE standards (overall AUC and Cmax), but still differ during certain time periods. OGD and the generic industry need tools to evaluate whether these differences observed in the generic could impact its therapeutic performance in comparison to the RLD.
- Generic modified release products may use a different release controlling mechanism than the RLD. The release mechanism can impact drug-drug interactions (DDI), the ability to split tablets or dose by sprinkling, or otherwise alter the substitutability when given to patients. OGD and the generic industry need tools that can assess the risks related to change in release mechanism.
- For MR drug products with multiple strengths, developing a product line with consistent in vivo performance across the strengths may need a more sophisticated formulation design than the simple proportionality than that is commonly used for IR products. OGD and the generic industry need tools to predict in vivo performance across strengths.
Research
OGD research combines physiological based pharmacokinetic (PBPK) and pharmacokinetic/pharmacodynamic (PK/PD) approaches to predict the impact of formulation changes on the resulting PK profiles and their therapeutic performance. To link the models to in vivo performance OGD conducts replicate design BE studies to evaluate if new study designs or analysis methods can aid MR BE reviews and has several ongoing PD and clinical comparisons of modified release products. Collaboration with FDA labs provides in vitro data to support these efforts and identify in vivo predictive dissolution methods to improve IVIVC development. Model based analysis informs recommendation on when to include partial area under the curve (pAUC) in BE guidances and helps OGD’s review process manage the risks related to MR products.
OGD research on MR products also includes analysis of product quality data and adverse event reports. Postmarketing surveillance based on the FDA Adverse Event Reporting System (FAERS) explores the substitution issues for MR drug products in different therapeutic classes and analysis of field alert quantifies reports of dissolution failures for MR relative to IR products. These data help identify the products with higher risk.
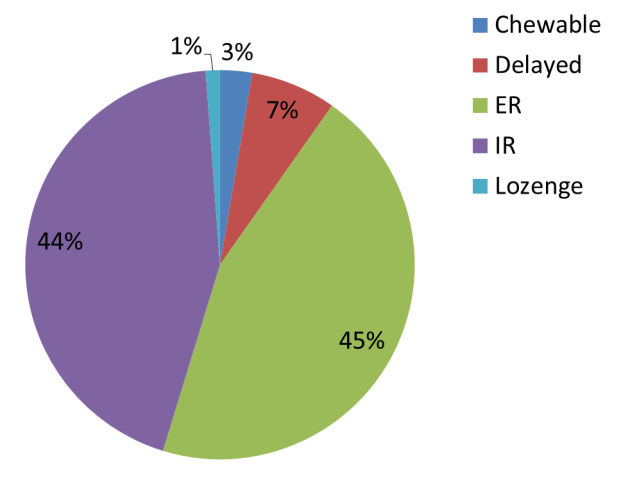
Figure 14. Distribution of dissolution failure in the FDA field-alert reports for various oral solid dosage forms from January 2005 to June 2014
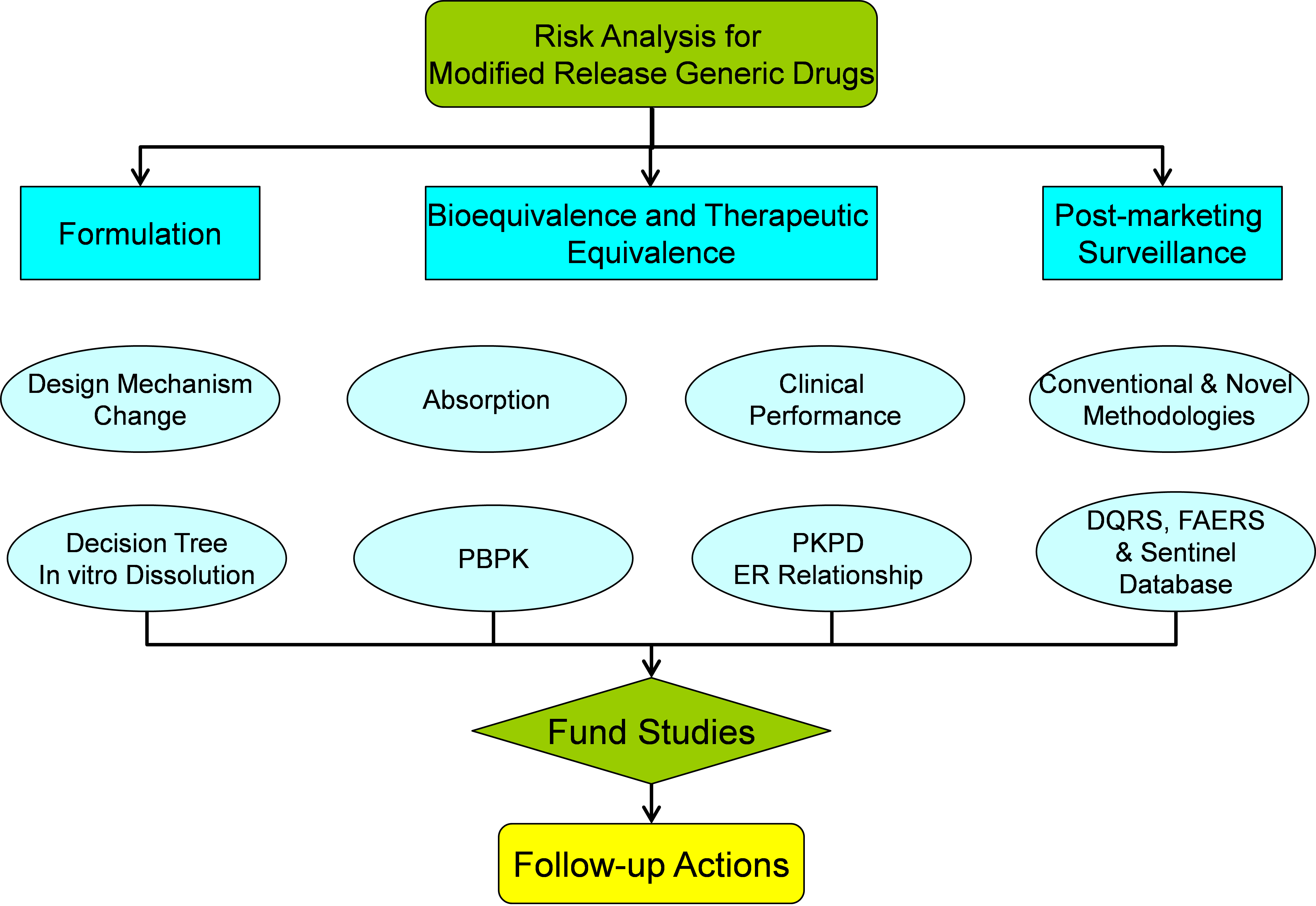
Figure 15. Strategy for risk analysis of MR drug products
ORS staff facilitating research in this area
- Hong Wen, Lanyan Fang, Xinyuan Zhang, Andrew Babiskin, Nan Zheng, Sarah Dutcher, Dajun Sun, and other ORS staff members
Projects and Collaborators
- Bioequivalence study of lamotrigine extended release tablets in healthy subjects
- Site PI: Bradley Vince
- Contract #: HHSF223201210030I
- Comparative surveillance of generic drugs by machine learning (AED drugs)
- Site PI: Peggy Peissig
- Contract #: HHSF223201510112C
- BE and characterization of generic drugs (methylphenidate)
- Site PI: Bradley Vince
- Contract #: HHSF223201210030I, HHSF22301001T
- Open-labeled pharmacokinetic and pharmacodynamic (PK-PD) studies of Metoprolol ER
- Site PI: Larisa Cavallari
- Grant #: 1U01FD005235-01
- Bioequivalence and clinical effects of generic and brand Bupropion
- Site PI: Evan Kharasch
- Grant #: 1U01FD004899-01
- A pharmacokinetic/pharmacodynamic study of methylphenidate formulations in pediatric attention-deficit/hyperactivity disorder (ADHD) patients in a laboratory classroom
- Site PI: Thomas Spencer
- Grant #: 1U01FD005240-01
- Internal Project: Dissolution testing and other in vitro analysis of marketed MR drug products
- FDA Collaborator: Zongming Gao, John Kauffman
- FDA Center/Office/Division: CDER/OPQ/OTR/DPA
Publications and Presentations
- Dajun Sun, Hong Wen, Anna Externbrink, Zongming Gao, John Kauffman, Lucinda Buhse, Gregory Krauss, Robert Lionberger, Wenlei Jiang. Ghost-Pill-Buster: A Case Study of Intact Levetiracetam Extended-Release Tablets in Feces. CNS Drugs, Accepted, 2016.
- 2015 AAPS Annual Meeting and Exposition, Orlando, FL (Oct. 2015)
- Developing Analytical Methods for In Vitro Comparative Nasogastric (NG) Tube Studies of Esomeprazole Magnesium Delayed Release Capsules. Alicia S. Hoover; Dajun Sun; Hong Wen; Wenlei Jiang; Minglei Cui; David Keire; Changning Guo.
- Dissolution testing of extended release matrix tablets in compendial and biorelevant media. Anna Externbrink, Wenlei Jiang, Hong Wen and Zongming Gao.
- 2014 AAPS Annual Meeting and Exposition, San Diego, CA (Nov. 2014)
- Survey of Failed Dissolution Related Field Alerts of Drug Products. Hong Wen, Mark Browning, Wenlei Jiang, and Rick L. Friedman.
Outcomes
- Research projects in progress
- Evaluated the necessity of pAUC for multiple MR drug products and updated product specific guidances when needed
- Contributed to BE reviews and approval of MR drug product ANDAs