FY2015 Regulatory Science Research Report: Advances in Predictive Dissolution and Physiological Models of Drug Absorption
Introduction
The majority of generic drugs in use are orally administered dosage forms such as tablets, capsules, and oral suspensions. For this core product category, GDUFA regulatory science supports regulating products more efficiently and developing products at a faster rate. Predictive dissolution methods and improved physiological models of oral drug absorption enable waivers of in vivo BE studies, support in vitro BE methods for locally-acting GI drugs, and aid the pharmaceutical development of bioequivalent generic products.
Research
Because of a critical knowledge gap for models that predict BE, repeated dosing of products to the same subjects under in vivo data sets is required. These data sets help us understand the within subject variation that affects product substitution. A series of ORS-supported studies are generating new in vivo data that will support the next generation of in vitro BE methods and improve the current models of oral drug absorption. With the University of Michigan, we are modeling GI fluid hydrodynamics, sampling GI tract fluids for their composition and pH, testing novel dissolution methods, and using in vivo PK studies to validate model predictions. With Purdue University, we are obtaining in vitro and in vivo data on solid dispersions that are currently not well predicted either by dissolution or in existing simulation tools. Studies at the University of Michigan collect in vivo data in the same subjects for multiple formulations of mesalamine and bupropion. The mesalamine data also include GI sampling and an IR arm. The bupropion data will help to explain the link between GI exposure and metabolism. Research just completed at the University of Maryland Baltimore generated new in vivo data on Biopharmaceutical Classification System (BCS) Class 3 drugs that will help us understand the effects of common excipients on drug absorption. Additional new in vivo data will originate from a study of fresh and aged warfarin.
Integration of these data will lead to biowaivers for BCS Class 3 drugs and more efficient pharmaceutical development of generic drugs due to more reliable predictions of in vivo performance. BE guidances for locally-acting GI drugs are outgrowths of this research area.
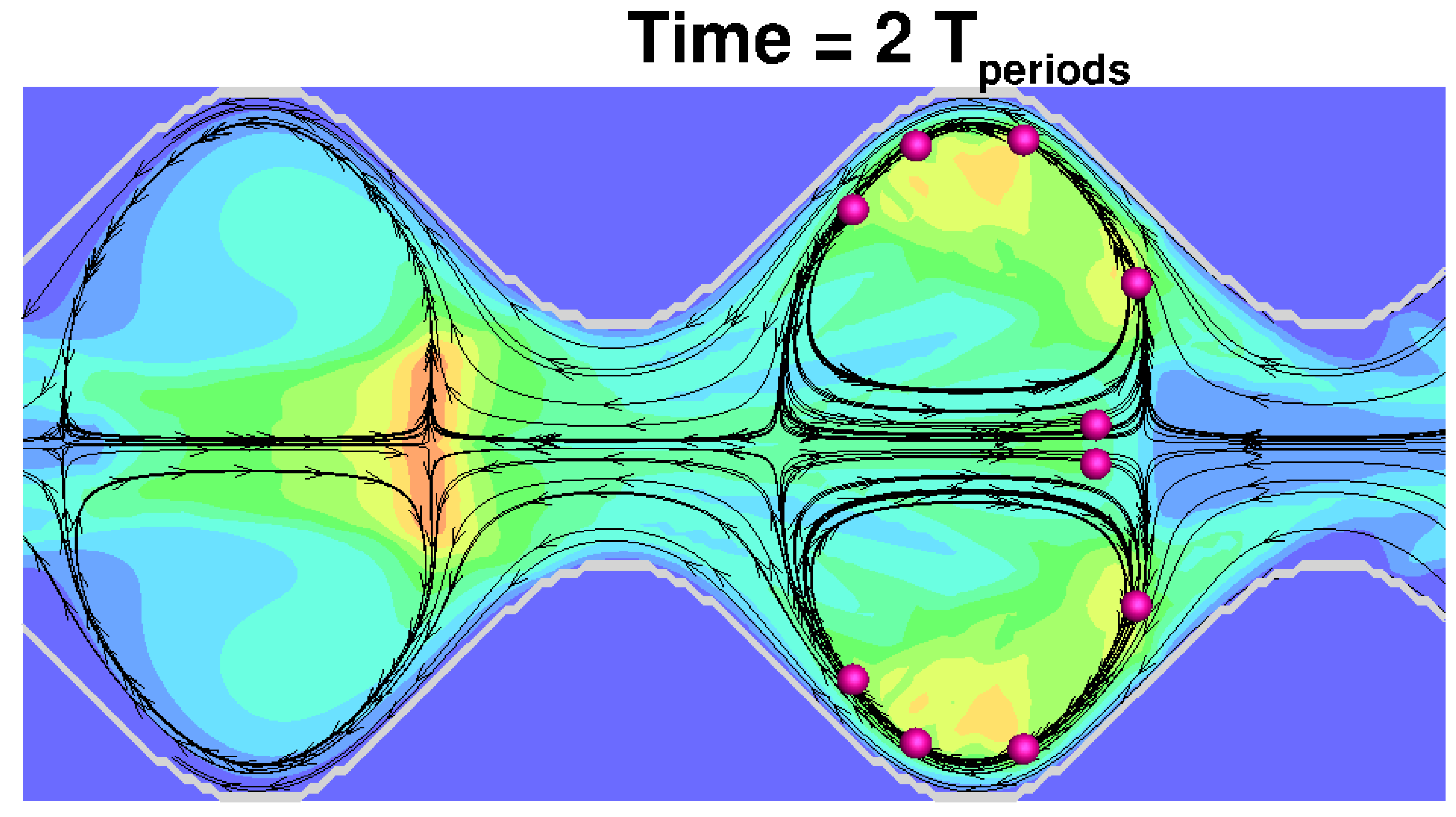
Figure 21. Modernization of in vivo-in vitro oral bioperformance prediction and assessment
Time=2T periods
Source: Brasseur J. et al. Modernization of in vivo-in vitro oral bioperformance prediction and assessment. Annual Report (2014)
ORS staff facilitating research in this area
- Xinyuan Zhang, Hong Wen, Hopi Lin, Arjang Talattof, Ming Qin, and other ORS staff members
Projects and Collaborators
- Effect of different preparation methods on the in vitro and in vivo performance of solid dispersion formulations
- Site PI: Lynne Taylor
- Grant #: 1U01FD005259-01
- Prediction of in vivo performance for oral solid dosage forms. Gordon Amidon, University of Michigan
- Site PI: James Brasseur
- Contract #: HHSF223201310144C
- Pharmacokinetic study of bupropion hydrochloride products with different release patterns
- Site PI: Duxin Sun
- Contract #: HHSF223201310164C
- Correlation of mesalamine pharmacokinetics with local availability
- Site PI: Duxin Sun
- Contract #: HHSF223201300460A
- Warfarin bioequivalence study
- Site PI: Bradley Vince
- Contract #: HHSF223201210030I
- Excipients’ impact on bioavailability of BCS Class 3 drugs
- Site PI: Jim Polli
- Contract#: HHSF223200910020C
Publications and Presentations
- Dajun Sun, Hong Wen and Lynne S. Taylor. Non-sink dissolution conditions for predicting product quality and in vivo performance of supersaturating drug delivery systems. Journal of Pharmaceutical Sciences, Accepted (2016)
- Wang et al. Analysis of diffusion-controlled dissolution from polydisperse collections of drug particles with an assessed mathematical model. J Pharm Sci (May 18, 2015)
- Tsume et al. In vitro dissolution methodology, mini-gastrointestinal simulator (mGIS), predicts better in vivo dissolution of a weak base drug, dasatinib. Eur J Pharm Sci (Aug 30, 2015);76:203-12
- Talattof et al. Fasted-state motility-dependent gastric emptying and plasma level variation: bioequivalence implications. AAPS Conference, San Diego CA (2014)
- Vaithianathan S et al. Impact of excipients on BCS Class 3 oral drug absorption. AAPS Conference, San Diego, CA (2014)
- Vaithianathan et al. Effect of Common Excipients on the Oral Drug Absorption of Biopharmaceutics Classification System Class 3 Drugs Cimetidine and Acyclovir. J Pharm Sci. (Sep 16, 2015)
Outcomes
- Research projects in progress