Outbreak Investigation of E. coli O157:H7: Unknown Food (Fall 2020)
FDA announces completion of two E. coli outbreak investigations.
The FDA and CDC, in collaboration with state and local partners, have completed the investigation on two of three multistate outbreak of E. coli O157:H7 infections in the U.S. this fall.
One of these investigations, Outbreak Unknown Source 3, identified 18 reported illnesses in nine states: California, Colorado, Illinois, Michigan, New York, Ohio, Pennsylvania, Virginia, Washington.
FDA completed a traceback investigation of several potential food vehicles identified in patient interviews and although no single farm was identified as a common source of the outbreak, FDA and state partners also conducted on-site investigations on farms of interest. However, information and samples collected in these inspections did not link these farms to the outbreak. The investigation of a farm does not mean that the farm is linked to an outbreak. The results of an investigation into a farm may well lead to that firm being ruled out of the investigation. On 12/18/2020, the CDC announced that this outbreak had ended.
The other completed outbreak investigation, Outbreak Unknown Source 1, identified 32 reported illnesses in 12 states: California, Illinois, Louisiana, Maryland, Michigan, Montana, New Jersey, Ohio, Utah, Virginia, Washington, Wisconsin. This strain of E. coli is genetically similar to a strain linked to a romaine outbreak that occurred in the spring of 2018, though a food was not linked to the current outbreak. FDA completed a traceback investigation and was unable to determine a common source of the outbreak. FDA and state partners also conducted on-site inspections on farms of interest, though information collected in these inspections did not link these farms to the outbreak. On 12/18/2020, the CDC announced that this outbreak had ended.
Investigations of a third E. coli outbreak of Unknown Source 2 continue.
Recommendation
Consumers, restaurants, and retailers, were advised not to eat, sell, or serve recalled Tanimura & Antle, Inc. brand packaged single head romaine lettuce with a pack date of 10/15/2020 or 10/16/2020.
The recalled products are now well beyond expiration and likely no longer on the market or in consumers’ homes.
Product Images
Recall Information
On November 6, 2020, Tanimura & Antle, Inc. recalled single head romaine lettuce under the Tanimura & Antle brand, labeled with a packed on date of 10/15/2020 or 10/16/2020, due to possible contamination with E. Coli O157:H7. Packages contain a single head of romaine lettuce with the UPC number 0-27918-20314-9.
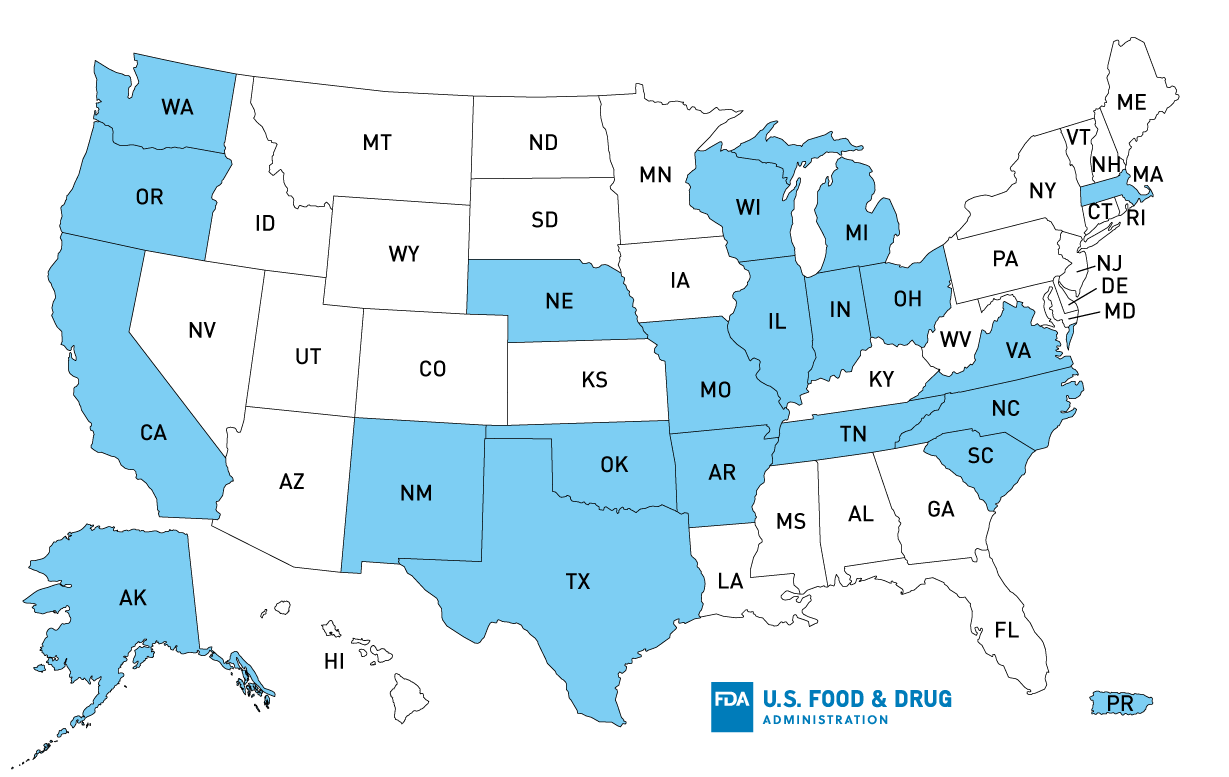
According to Tanimura & Antle, potentially affected product was distributed to the following states: AK, OR, CA, TX, AR, OK, IN, NE, MO, TN, WI, NM, SC, WA, NC, OH, VA, MA, PR, and IL, but product could have been further distributed, reaching additional states, including MI.
Product was shipped in cases packed in either 12, 15, 18 or 24 heads per case. Retailers and distributors can identify the potentially affected products through the Produce Traceability Initiative (PTI) sticker attached to exterior of the case. The PTI codes are 571280289SRS1 and 571280290SRS1.
U.S. Distribution Map of Recalled Tanimura & Antle, Inc. Single Head Romaine Lettuce
Product Distribution*: AK, OR, CA, TX, AR, OK, IN, NE, MI, MO, TN, WI, NM, SC, WA, NC, OH, VA, MA, PR, IL
*States with confirmed distribution; product could have been distributed further
Case Counts
For these outbreaks, case counts are found on CDC’s website:
- CDC's Outbreak Page: Unknown Source 1
- CDC’s Outbreak Page: Unknown Source 2
- CDC’s Outbreak Page: Unknown Source 3
Useful Links
- Food Safety Tips for Retailers and Consumers During an Outbreak
- Food Safety Resources for Produce Shippers and Carriers During a Foodborne Illness Outbreak
- Who to Contact
- What is E. coli?
- CDC's Outbreak Page: Unknown Source 1
- CDC’s Outbreak Page: Unknown Source 2
- CDC’s Outbreak Page: Unknown Source 3
- Michigan Consumer Advisory: Romaine lettuce grown by Tanimura & Antle tests positive for E. coli
- Recall Notice: Tanimura & Antle Voluntary Recalls Packaged Single Head Romaine Lettuce Due to Potential E. Coli O157:H7 Contamination
Previous Updates
November 12, 2020
The FDA and CDC, in collaboration with state and local partners, is investigating illnesses in a third multistate outbreak of E.coli O157:H7 infections this Fall.
On November 6, 2020, the Michigan Department of Agriculture and Rural Development (MDARD) reported that as a part of routine sampling, they collected a product sample of romaine lettuce for testing. The sample tested positive for E. coli O157:H7 and subsequent whole genome sequencing (WGS) analysis determined that the E. coli O157:H7 present in the samples matches the strain that has caused illnesses in this outbreak.
The strain of E. coli found in the Michigan sample is a third distinct strain not genetically related to the strains causing two distinct multi-state outbreaks of Shiga-toxin producing E. coli O157:H7 (STEC) that FDA and CDC announced on October 28, 2020. At this time, a specific food has not been linked to either of those outbreaks.
On November 6, 2020, Tanimura & Antle, Inc. recalled packaged single head romaine lettuce with a pack date of 10/15/2020 or 10/16/2020 due to possible contamination with E. coli O157:H7. The firm recalled this product based on test results from a product sample collected and analyzed by MDARD before the WGS analysis showing the match to the outbreak strain was completed.
FDA and state partners are working with the firm to determine if additional romaine should be recalled.
At this time, there is not enough epidemiologic and traceback evidence to determine if ill people in this outbreak were exposed to romaine lettuce from Tanimura & Antle, Inc. Additional information will be provided as it becomes available.
Who to Contact
Consumers who have symptoms should contact their health care provider to report their symptoms and receive care.
To report a complaint or adverse event (illness or serious allergic reaction),
visit Industry and Consumer Assistance.
Follow us on X (formerly Twitter)