MPM: V-11. Vegetables and Vegetable Products
Macroanalytical Procedures Manual (MPM) Main Page
Contents
- Method for Asparagus
- Method for Brussels Sprouts
- Method for Microscopic Detection Of Substitute Vegetable Tissues in Ground Horseradish
- Method for Lettuce
- Method for Mushroom Products
- Method for the Preservation and Identification of Mushrooms
- Method for Peas and Beans (Canned, Frozen, and Dried)
- Method for Pickled Vegetables and Relish
- Method for Pimientos
- Method for Potato Chips
- Method for Corn Husks
- Method for Garlic Bulbs
A. Method for Asparagus (V-93)
(1) Scope
This method covers procedures for visual examination of individual shoots or pieces of asparagus to detect damage due to the asparagus beetle and other insects.
The succulent young shoots of this widely cultivated vegetable, Asparagus officinalis L., are cooked and consumed in a variety of recipes. The color of the shoots varies from white to dark green.
(2) Applicable Documents
- CPG 7114.02 Defect Action Levels
- 21 CFR 155.200 Standard of Identity
(3) Defects
Asparagus beetles are major pests of asparagus. There are two species, the asparagus beetle [Crioceris asparagi (L.)] and the spotted asparagus beetle [Crioceris duodecimpunctata (L.)]. The asparagus beetles emerge in the spring about the time asparagus shoots appear. The young tips are damaged by beetle feeding and egg laying. The eggs are normally dark in color and attached singly on end by a secretion which forms a hardened base between the egg and the spear or shoot. The base may remain after the egg sac has been removed. After the egg has hatched, the egg sac may remain attached and appear as a dark spot on the spear.
Larvae of the armyworm [Pseudaletia unipuncta (Haworth)] have been found on imported frozen asparagus. The larvae range in size from 2 to 3 mm in length and have been reported to reach a maximum length of 35 mm.
Thrips have also been reported to be a pest of asparagus.
(4) Procedure: Determination of Asparagus Beetle Eggs and Egg Sacs in Asparagus
- Sample Preparation -- Determine drained weight of canned asparagus and net weight of frozen product. For canned asparagus, pour contents of can evenly over a weighed No. 2 sieve. Use 8-in. sieve for containers of less than 3 lb net weight and 12-in. sieve for larger containers. Drain for 2 min and reweigh sieve and asparagus to determine drained weight of asparagus. Rinse container. Combine drained liquid with rinsing and set aside for use in Procedure (5)a.
-
Visual Examination and Classification of Rejects -- Place contents of can or thawed frozen package in a shallow white pan and cover with water. Roll each spear or piece and count the beetle eggs and egg sacs attached to it. Magnification may be used if needed. Class as a reject any spear or piece having six or more attached beetle eggs and/or egg sacs.
Any whole insects or equivalent, including thrips, detected by this examination, should be counted and combined with the results obtained in (5).
- Report -- Report the percentage of reject spears or pieces by count.
(5) Procedure: Determination of Insects in Asparagus
- Examination of Drained Liquid -- Filter drained liquid and rinsing from (4)a. through ruled filter paper; examine and record separately "whole insects and equivalent" and thrips. Do not count cast skins of thrips.
-
Examination of Drained Asparagus -- Insert 7-in. funnel containing a 5-in. No. 12 sieve into a 2 L Wildman trap flask. Decant liquid contents of pan containing asparagus from (4)b. through sieve into trap flask. Cover asparagus in pan with water and wash by stirring to release any insects. Decant liquid again through sieve to trap, flask. Repeat washing and decantation to trap flask. Extract, trap, and filter, using water and 25 and 15 mL portions of n-heptane (III. (7)). If pieces of asparagus float in the heptane, remove with forceps and rinse over the filter with water. Examine papers microscopically and record the number of whole insects or equivalent according to categories in AOAC 970.66B(i) and the number of thrips. Do not count cast skins of thrips. Calculate the number of thrips per 100 g of asparagus as follows:
No. thrips in
drained liquid
((5)a.)+ No. thrips in
drained asparagus
((5)b.)x 100
wt (g) drained
asparagus - Report -- Report total number and breakdown of whole insects and equivalent for each container or subsample and the average of the subsamples. Report also the number of thrips in 100 g asparagus from each container or subsample examined. Also report the average of the subsamples.
B. Method for Brussels Sprouts (V-95)
(1) Scope
Brussels sprouts are prepared from the clean, sound, succulent heads of the Brussels sprouts plant (Brassica oleracea L. var. gemmifera). This method contains a procedure for determination of whole insects and equivalent by visual examination of a representative sample after cutting the sprouts to expose the presence of aphids, thrips, or other insects.
(2) Applicable Documents
(3) Defects
The major defects associated with brussels sprouts are contamination by insects such as aphids and thrips.
(4) Procedure: Determination of Insects in Brussels Sprouts
- Sample Preparation and Visual Examination -- If frozen, thaw the sample and weigh at least 100 g per subsample. Cut heads longitudinally into quarters and immerse in water in a white pan. Spread leaves to expose insects. Count all aphids and thrips on the heads and those loose in the water that are visible without magnification. Magnification may be used to distinguish between whole aphids and thrips and cast skins. Do not count cast skins of aphids and thrips.
- Report -- Report the number of aphids and thrips in each subsample and the average number per 100 g. Report separately other types of insects.
C. Method for Microscopic Detection Of Substitute Vegetable Tissues in Ground Horseradish (V-96)
(1) Scope
This method describes a microscopic procedure for detecting and estimating the amount of foreign tissues or adulterants added to or substituted for horseradish root in the preparation of horseradish sauce.
Horseradish sauce is prepared by crushing, mincing, or powdering the root of Armoracia rusticana Gaertn., Mey. & Scherb., a member of the Cruciferae family. In processing horseradish, the roots are washed in a tumbler or rotary washer, and either scraped to remove the brown outer skin, or cleaned in an automatic vegetable peeler prior to grinding.
(2) Applicable Documents
(3) Defects
A few manufacturers have occasionally prepared imitation horseradish products from cheaper substitutes without declaring such on the label. Parsnip and turnip roots have been encountered most frequently as substitutes for the genuine horseradish root. The microscope offers a ready means for detecting such adulterants, based on diagnostic histological characteristics which distinguish horseradish root tissues from substitute materials.
(4) Procedure: Microscopic Determination of Substitute Vegetable Tissues in Ground Horseradish
-
Diagnostic Microscopic Features of Ground Materials
- (i) Horseradish -- Horseradish starch grains are elliptical to nearly spherical with indistinct central hilum. The grains are generally 3-15 micrometers in diameter, with some grains occasionally reaching 25 micrometers. Elongated xylem and phloem parenchyma cells measure 100 to 150 micrometers in length and have pointed ends that lock together with the adjacent cells. Irregularly rounded to greatly elongated stone cells are scattered throughout the cortex.
- (ii) Parsnip -- Parsnip starch grains are irregularly rounded and polygonal. The grains are 3 to 7 micrometers in diameter. Some grains occasionally reach a diameter of 15 micrometers. A cross-hatched appearance of the xylem rays is quite characteristic in longitudinal sections. Oil is especially vivid after staining with Sudan III and warming in acidified chloral hydrate glycerol solution.
- (iii) Turnip -- Turnip cells are large, thin-walled and isodiametric. They are devoid of starch. The cells appear in cobweb-like groups.
- Microscopic Examination -- Mount a small portion of the subsample in water and examine microscopically for foreign starch. Place about 5 g of the subsample in a 50 mL test tube, add 5 mL I-KI solution, and shake until thoroughly mixed. Let soak for 5 min to stain the horseradish starch thoroughly. Place in a No. 60 sieve and rinse with a stream of water to remove excessive I-KI solution. Transfer the stained material to a petri dish, cover with a small amount of water and examine with a widefield binocular microscope. Horseradish tissues are stained a deep blue. Parsnip roots may stain either a light or dark blue since the amount of starch present will vary depending on whether roots used were dug in the spring or fall. Turnip and other starch-free roots will stain yellow. If a considerable number of yellow-stained fragments are present, one may suspect the addition of turnip or parsnip to the product. Make microscopic mounts in acidified chloral hydrate-glycerol solution of several fragments and check identity from the diagnostic characteristics listed in (4)a. above. Estimate the approximate percent of foreign tissues present by determining identity of 25 or more pieces of tissue selected at random. More accurate estimates of amounts of foreign tissues present may be made by comparing the subsample material with authentic mixtures containing known percentages of each ingredient in the mixture.
- Report -- State the presence and approximate percent (by weight) of tissues other than horseradish found.
REFERENCES
Ballard, Chas. W. and F. J. Pokorny, "Histological Study of Horseradish Root and Some Common Adulterants," J. Am. Pharm. Assoc. 28: 376-381, June 1939.
Winton, A. L., and K. B. Winton, "Vegetables, Legumes and Fruits," Structure and Composition of Foods, Vol. 2, pp. 72-75 , John Wiley and Sons Inc., New York, 1935.
D. Method for Lettuce (V-98)
(1) Scope
This method covers a procedure for the visual examination of lettuce to determine the percentage of leaves in the head that are either decomposed and/or contaminated with insects. The method employs a washing and separation process to determine numbers of insects in a representative sample.
Numerous varieties of lettuce are under cultivation; they are grouped as either
- Common
- COS or romaine.
Garden lettuce is derived from Lactuca sativa L. and is used primarily as a salad ingredient. Leaves of common lettuce vary in color from light to dark green. Leaves of some species are mottled with brown or purple.
(2) Applicable Documents
(3) Defects
Bacterial spot of lettuce is caused by Xanthomonas vitians, a bacterium which forms raised, pale-yellow colonies on nutrient dextrose agar. Infestation takes place through stomata during periods of high humidity, dew, and rain. The disease is characterized by circular or irregular translucent water-soaked leaf lesions which become dark brown with age. If the infection is severe it may render the crop unmarketable.
Bacterial soft rot, often called "slime," is the most serious market disease of lettuce. The common causative agents are Pseudomonas spp. and Erwinia spp. In the early stages of infection the tissue appears to be water-soaked and may develop a russet or brown color. As the decay progresses the lettuce becomes soft and slimy. Under dry conditions, decayed areas of the outer leaves may become papery in texture.
Aphids and other insects such as leaf miners also may infest lettuce.
(4) Procedure: Examination of Lettuce for Decomposition and Insect Contamination
- Sample Preparation -- Select at least three heads from each subsample or select a minimum of nine heads from each sample. Weigh the heads from each subsample. Strip off and discard any obviously worthless outside leaves.
-
Visual Examination and Report -- Examine the prepared heads by stripping off single leaves and examining each for decomposed areas, aphids, and other insects. For each head, report the total number of leaves and the number showing rot spots in. or greater in diameter. Calculate and report the percentage by number of leaves with rot.
Report the percentage by number of leaves in each subsample (three or more heads) showing aphid or other insect infestation and state whether the insects are alive or dead. Wash each insect-infested leaf with water, using an aerator [AOAC, 945.75B(a)]. Pour the combined washings from each subsample through an 8 in. No. 80 sieve. Transfer residue from sieve to ruled filter. Examine at about 15X. Report the total number of aphids, aphid heads, and other insects and calculate the number of each per 100 g of each sub-sample.
State whether the rot and/or insect infestation is general throughout the head or confined to the outer leaves.
REFERENCE
U.S. Department of Agriculture Handbook No. 155, Washington, DC, April 1959.
E. Method for Mushroom Products (V-100)
(1) Scope
The common cultivated mushroom (Agaricus bisporus) is sold commercially as a fresh, canned, frozen, and dried, product. A method for the determination of maggots and mites in canned, fresh, frozen, freeze-dried and dehydrated mushrooms is specified in AOAC 967.24A. In addition, AOAC 967.24B provides a procedure for detecting light and heavy filth in dried (not powdered) mushrooms. To supplement the foregoing, this method includes procedures for mushroom products which cover
- Determination of damage by decomposition (mold, etc.) in individual canned, dried, and fresh mushrooms
- Determination of light filth and maggots in mushroom soup
(2) Applicable Documents
(3) Defects
- Insect Infestation and Damage -- Two insects commonly infest mushrooms. The mushroom fly larva (Lycoriella spp.) is a black-headed maggot which tunnels through the stem and cap of the mushroom. The larvae of the Cecidomyiid or cecid fly, which feed primarily around the mushroom gills, are pointed at both ends, and reach a length of 3 mm. Less commonly, maggots of the phorid fly have also been found in canned mushrooms. In addition, several species of mites also attack mushrooms. Of these, the mold mite (Tyrophagus putrescentiae (Schrank)) is the most serious pest.
- Mold and Other Microbial Deterioration -- Several species of bacteria and fungi produce discolored rot areas on the mushrooms. These areas appear as dark brown or black spots, streaks, blotches, or pits. Verticillium mold infection can be recognized by the gray bloom which develops in the centers of the older spots or pits as a result of the growth of conidiophores. The mold Trichoderma is a much less common cause of spotting and pitting. Typically, Verticellium grows close to the surface of the mushroom, while Trichoderma penetrates more deeply into the tissues, often causing large portions of the mushroom to become discolored and rotten.
(4) Procedure: Determination of Damage by Decomposition in Canned, Dried, and Fresh Mushrooms
- Sample Preparation -- For canned mushrooms, pour contents of can evenly over No. 8 sieve. Use an 8-in. sieve for containers of net weight less than 3 lb and a 12-in. sieve for larger containers. Drain 2 min or longer. For dried mushrooms, place about 20 g from each subsample into a suitable size container and add sufficient water to immerse the mushrooms. Soften mushrooms by soaking for several hr or, alternately, by heating on a steam bath or boiling 1.5 to 2 hr as necessary. Drain 2 min or longer on a No. 8 sieve. Use fresh mushrooms as is.
-
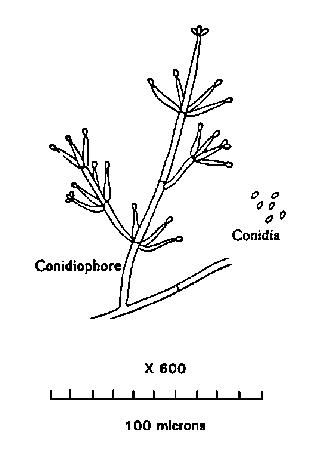
Visual Examination -- Weigh 100 g of the drained canned or dehydrated mushrooms and 200 g fresh mushrooms to be used as analytical units. Examine each mushroom or piece under good lighting for evidence of decomposition. Classify as a reject any mushroom or piece which contains at least one decomposed area 1/4 in. or more in average diameter. These areas will appear as dark brown or black spots, streaks, blotches, or pits (see (3)b. above). Examine the tissue from discolored areas microscopically for the presence of pathogens. If microscopic examination shows characteristic conidiophores of Verticillium, consisting of a central stalk with whorls of branches, the presence of Verticillium is confirmed (See Figure V-8). Separate and weigh the reject mushrooms and pieces.
FIGURE V-8
VERTICILLIUM CONIDIOPHORE AND CONIDIA AT 600X
- Report -- Report the percent (by weight) of reject decomposed mushrooms in each analytical unit. Also report the average percent (by weight) of rejects for all units examined.
(5) Procedure: Determination of Light Filth by Flotation in Mushroom Soup
-
Sample Preparation and Microscopic Examination -- Use the entire contents of a can (about 10 3/4 oz.) as the analytical unit. Weigh and pour the entire contents of the can into 1.5 L beaker. Bring the volume to 900 mL with hot tap water. Add the following and stir well:
- 100 mL HCl
- 1 sec. spray of Antifoam A [AOAC 945.75C(e)]
- 10 mL Igepal CO-730 [AOAC 945.75C(j)].
Bring to a boil and continue boiling with rapid magnetic stirring for 30 min. Pour a small portion at a time onto a No. 230 sieve and wet-sieve with hot tap water. Wash each portion on the sieve with 40% isopropanol and transfer to a 2 L trap flask. Bring the volume to 1 L with 40% isopropanol, add 50 mL HCl and boil 10 min with gentle magnetic stirring. Transfer the trap flask to a cool magnetic stirrer, add 40 mL light mineral oil, and stir at maximum speed (without visible or audible splashing) for 3 min. Fill with water and trap off into a beaker. Stir 30 mL light mineral oil into the flask and perform second trapping after 10 min. Filter combined trappings from beaker and examine microscopically. Retain the trap flask contents for further analysis as in (6) below.
- Report -- Report, using the applicable categories in AOAC 970.66B(i).
(6) Procedure: Determination of Maggots in Mushroom Soup
- Sample Preparation and Microscopic Examination -- Pour the settled residue in trap flask from (5)a. above onto a No. 230 sieve and rinse thoroughly with isopropanol and hot tap water. Quantitatively transfer residue on sieve to a 600 mL beaker. Proceed as in AOAC 967.24A(a), paragraph 3, beginning with, "Pour mixt. into nested set of 8" Nos. 20, 40 and 140 sieves . . . ."
- Report -- Report the number of maggots for each analytical unit and the average number per unit weight.
F. Method for the Preservation and Identification of Mushrooms (V-102)
(1) Scope
This method describes a procedure for preserving specimens of mushrooms and related fungal products for specific identification by a qualified taxonomist. This is important when toxic or non-edible species may be suspected as contaminants in a product.
At present, there are at least 17 edible species of fungi being cultivated commercially. The "mushroom" most familiar to the American consumer is Agaricus bisporus. Another species, Agaricus bitorquis, which closely resembles A. bisporus, is rapidly gaining in world economic importance. The tolerance of A. bitorquis for higher cultivation temperatures permits its widespread cultivation above ground. Other cultivated mushrooms available to the consumer include
- Volvariella volvacea -- the Padi straw mushroom
- Lentinus edodes -- the shiitake mushroom
- Pleurotus ostreatus (Jacq) Fr. -- the Oyster Mushroo m.
Wild European mushrooms available in either canned or dried form include:
- Morchella spp. -- morels
- Cantharellus cibarius Fr. -- Chanterelles
- Boletus spp. -- cepe or steinpiltz.
(2) Applicable Documents
- Import Alert 25-02-Morels
(3) Defects
Several toxic and potentially lethal species of fungi have been found as contaminants in imported products. Among these are the following:
- Verpa Bohemica (false morels)
- Gyromitra spp. (false morels)
- Helvella spp.
(4) Procedure: Method for Preserving Mushrooms for Taxonomic Identification
Mushroom identification requires extensive training and experience and should not be attempted by an inexperienced analyst. There are a variety of pictorial keys and handbooks available on mushroom identification; however, these are useful only to the specialist or experienced taxonomist. A combination of authentic specimens, direct microscopic examination, spore prints, and microchemical tests are sometimes necessary to differentiate edible and toxic mushrooms.
To preserve specimens, dry them with forced warm air (40-50C). Chemical preservatives such as alcohol or formalin may be used, but a portion of the unknown mushroom or sample should also be dried.
REFERENCES
(1) Miller, Orson K., Mushrooms of North America, Dutton, New York, NY, 1979.
(2) Singer, Rolf, The Agaricales in Modern Taxonomy, 3rd Ed., J. Cramer Verlag, Braunscheig, Germany, 1975.
G. Method for Peas and Beans (Canned, Frozen, and Dried) (V-104)
(1) Scope
This method covers procedures for the preparation and visual examination of canned, frozen, and dried peas and beans for mold and insect damage. Legumes or pulses (Family Leguminosae) are grown for their edible seeds. While many varieties of legumes are referred to as beans and peas, there is at least one variety of legumes, lentils, which is not. Types of peas and beans covered by this method include but are not limited to
- Beans of the genus Phaseolus, such as French, "string," "snap," or kidney beans (Phaseolus vulgaris L.); the lima or butter bean (P. lunatus L.);* and the scarlet runner bean (P. coccineus L.)
- Beans of the genus Vigna such as mung beans [Vigna radiata (L.) Wilczek], the species from which bean sprouts are produced; the adzuki bean [Vigna angularis (Willd.) Ohwi and Ohashi], similar but with larger seeds than the mung bean, and which is red, cream, lilac, or mottled in color
- The large podded or broad bean (Vicia faba L.) also called "lupini bean"
- Soybeans and soya seeds [Glycine max (L.) Merr.] used in such products as processed soybeans, processed soybean oil, soy flours, fermented soy products, soy mills, bean cake, and tofu.
- The true pea (Pisum sativum L.)
- Cowpeas, black-eyed peas, crowder peas, field peas, and southern peas [Vigna unguiculata (L.) Walp. subsp. unguiculata]
- Yard-long bean (Vigna unguiculata subsp. sesquipedalis (L.) Verdc.)
- Pigeon pea (Cajanus cajan (L.) Huth)
- Chick pea, garbanzo or Bengal gram (Cicer arietenum L.), which are rounded seeds with a characteristic "beak" resembling the head of a baby chicken
- Lentil (Lens culinaris Medik.).
* White seeded types are usually grown because colored seeds often have a relatively high cyanogenic glycoside level, which can be dangerous.
(2) Applicable Documents
- CPG 7114.05 Lygus bug damage in dried cowpeas
- CPG 7114.11 Cowpea curculio in canned cowpeas
- CPG 7114.15 Insect damage and rocks in dried peas and beans
(3) Defects
-
Insect Damage in Canned and Frozen Peas and Beans -- Peas and beans may become infested by various types of insects, their eggs, and larvae. Such infestation may be found in the finished product.
Pea weevil infestation [Bruchus pisorum (L.)], by far the most commonly encountered defect in peas, occurs in the fields prior to harvesting. Adult weevils lay their eggs on the tiny pods, and after hatching, the larvae perforate the skins and enter the peas. Infestation is evidenced by the presence of small round holes on the peas. These holes are usually accompanied by discolorations around the openings. The larvae may be found under the skin embedded in the cotyledon.
The bean weevil [Acanthoscelides obtectus (Say)] may infest beans in the field, although it is also a serious pest of dry beans in storage. The larvae attack the seeds of several varieties of beans (including kidney beans, limas, and cowpeas) in the field and in storage. It also attacks faba beans, peas, lentils, and others in storage only.
Cowpea curculio [Chalcodermus aeneus (Boheman)] is a common defect in field peas and black-eyed peas. Some variety of crowder peas and the California black-eye are more susceptible than other varieties. The greatest damage from this insect is done by the larvae feeding on the seed within the green pods. Shelled peas with curculio damage have small dark spots present, which may or may not contain an egg or larva. These small dark spots are commonly called "weevil stings." The curculio is legless and C-shaped. Its body is pale yellow and the head is brown. The larva is less than 1/4-in. in length when full grown.
-
Weevil and Moth Damage in Dried Peas and Beans -- There are five major insect species that attack dried peas and beans. They are the bean weevil (Acanthoscelides obtectus (Say)), the cowpea weevil (Callosobruchus maculatus (Fabricius)) the Indianmeal moth (Plodia interpunctella (HØbner)), the tobacco moth (Ephestia elutella (HØbner)) and the almond moth (Cadra cautella (Walker)).
The bean weevil and the cowpea weevil are the most destructive. They attack beans and peas while they are in the field, where they may become heavily infested before they are taken to the warehouse. These species will breed continuously in the dry seeds if stored in a warm place. The insect eggs of the bean weevil are smooth and white, and are laid singly or in clusters among or near the beans. The eggs hatch into tiny white larvae, which eat their way into the beans. The entrance holes are small and easily overlooked. In warm weather, the larvae develop rapidly within the beans and soon reach the pupal, or resting, stage from which the adults are formed. The adults then chew a round opening through the seed coat and emerge. The bean weevil is capable of developing in all varieties of common beans and cowpeas. The life cycle of the cowpea weevil is similar to that of the bean weevil, except that the eggs are glued to the pods or exposed seeds. The hatching larvae chew from the egg through the seed coat and into the seed.
The damage caused by the three species of moths that attack dry peas and beans results chiefly from the webbing and frass that is deposited among the beans and peas while the larvae are feeding. The larvae can feed only on beans and peas that are split or have cracked seed coats.
-
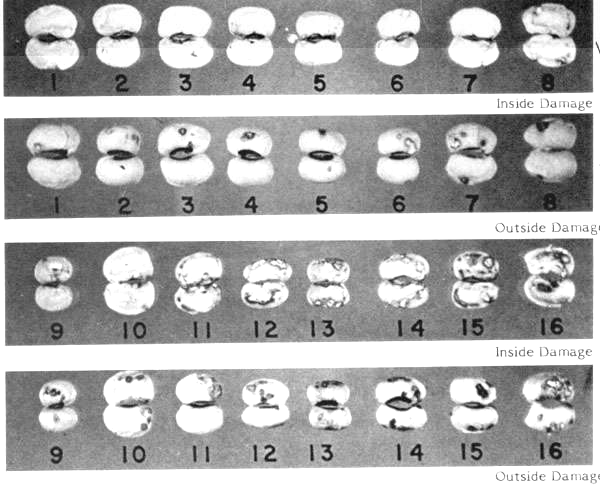
Lygus and Aphid Damage in Dried Peas and Beans -- In the field, peas and beans may also be damaged by sucking insects. The lygus bug (Lygus spp.) and the aphid may damage legumes by the insertion of the proboscis into the growing seed to inject a digestive juice and to suck out plant substances such as protein. This results in an area of dead tissue which appears as a brown spot or in some cases a chalky pit surrounded by brown scar tissue. In some instances the damage does not penetrate the cotyledons; however, in other instances there is extensive inner damage while only a small amount of damage can be detected on the exterior. Figure V-9 shows the type of interior damage done to black-eyed peas by the lygus bug and aphid. The illustrations have been arranged to show increasingly greater aphid and lygus bug type of damage.
FIGURE V-9
- Mold Damage in Dried Peas and Beans -- Dried beans with no visible external damage and an apparently intact integument (seed coat) may have extensive internal mold and require splitting of the cotyledons to detect.
(4) Procedure: Determination of Insect-Damaged Peas or Beans in the Canned or Frozen Product
Examine at least 200 peas or beans. In canned beans and peas, the insect plug or exit hole shows as a hole in the bean which is often light brown or black. In frozen beans, the insect damage is mainly due to the insect's egg being laid in the bean. This can often be seen in beans by staining them with a dilute iodine solution. The insect egg plug shows up as a small dot which is dark blue or black. Classify as insect-damaged any pea or bean containing an insect(s) or showing definite evidence of tunneling or frass. Report percent by number of insect-damaged peas or beans. If any appreciable amount of weevil damage is noted, proceed as in (5)c. below, as necessary.
(5) Procedure: Determination of Insect-Damaged Peas or Beans in the Dried Product
- Visual Examination for Weevil Damage -- Examine at least 12 subsamples. Cowpea weevil eggs appear as white oblong mounds approximately 0.4 X 0.7 mm that are firmly glued to the outer surface of the seed. Weevil egg punctures are very small but can occasionally be seen. Weevil exit holes are about 1 mm in diameter and show little or no discoloration. Classify as insect-damaged any pea or bean containing an insect(s) or showing evidence of tunneling or frass. Report percent by number of insect-damaged peas or beans. If any appreciable amount of weevil damage is noted, proceed as in (5)c. below, as necessary.
- Visual Examination for Lygus or Aphid Damage -- Examine at least 12 subsamples. In the case of lygus or aphid type of damage, classify as rejects all peas or beans which have exterior damage equal to or greater than No. 6 in Figure V-9. Split open those beans or peas having less exterior damage than No. 6, and examine for interior damage. When the interior and exterior areas of damage (as judged from the figure) are added together and the total divided by two is equal to or greater than No. 6, classify the bean or pea as a reject. Report number of beans or peas examined and number and percentage of rejects. >
- Examination for Internal Weevil Damage -- Proceed as in AOAC 945.81.
(6) Procedure: Determination of Moldy Peas or Beans in the Dried Product
- Sample preparation -- Examine at least 200 peas or beans. Soak beans 15-30 min in 70% alcohol or until seed coat separates from the cotyledons as evidenced by wrinkles in the seed coat. Remove beans or peas from the alcohol solution and place on absorbent paper. Gently split beans or peas with scalpel, hilum side down. The seed coat and the cotyledons will separate. Using a #5 or #7 jeweler's forceps, separate cotyledons and seed coat and place them in 100% isopropanol. Remove cotyledons and seed coat and place on a spot plate. They should dry in about 2 min.
-
Visual examination and Classification of Rejects -- Examine the internal surface of the seed coat for the presence of mold hyphae. In cases of extensive invasion, hyphae and fruiting bodies may be present between the cotyledons, and the cotyledons may be discolored.
Suspected hyphae and fruiting bodies may be confirmed by removing them with forceps, mounting in glycerol-alcohol (1 + 1 v/v) solution, and examining with the compound microscope at 100-200X. The characteristics of mold hyphae used in the Howard mold count method should be applied in confirming mold hyphae (AOAC Fig. 984.29B). Classify any pea or bean in which 1/4 or more of the internal surface of the cotyledons is affected by mold as mold-damaged.
- Report -- Report percent by number of mold damaged peas or beans.
H. Method for Pickled Vegetables and Relish
(1) Scope
This method covers procedures for detection of insects, grit, and mold in individual pickles and pickled vegetables.
The cucumber (Cucumis sativus L.) is commonly eaten raw or pickled, and is eaten less often as a cooked vegetable. Characteristics of the fruit are:
- The elongated, rounded triangular form
- The three locules
- The more or less prominent spiny warts
- A hard green rind
- Succulent flesh
Cucumbers are processed commercially into a variety of pickle products and relish.
(2) Applicable Documents
(3) Defects
Cucumbers may be contaminated by insects which enter the crop in the field. The pickleworm [Diaphania nitidalis (Stoll)], which is an internal feeder in cucumbers, may not be eliminated during the processing steps unless the pickle stock is adequately sorted or trimmed. Cucumbers may contain excessive grit, sand, or soil when delivered for processing. These contaminants may not be entirely removed by washing unless special care is taken.
Cucumbers and other vegetables are frequently pickled in open tanks; use of this method may allow insects and other animal filth to get into the finished product tank unless care is exercised to prevent contamination. Outdoor salt tanks may accumulate large numbers of various types of flying insects in the surface brine. Insects and other debris may also be found below the surface, due to periodic addition and circulation of brine. Indoor sweetening tanks are particularly attractive to drosophilids which breed on any exposed product and on the inner sides of the tanks above the level of the liquid.
Moldy cucumbers or other vegetables are sometimes used in pickled and chopped vegetable relishes. The moldy condition of the cucumbers or vegetables is concealed in the product by grinding or chopping.
(4) Procedure: Determination of Grit, Sand, and Soil in Pickled Vegetables
- Sample Preparation -- Examine at least 6 containers to provide a minimum of 100 pickles. Drain entire contents of each container on a No. 10 screen. Collect the brine and set aside for (4)c.
- Organoleptic Examination - Count and examine contents of each jar separately. Cut pickles other than gherkins and midgets into approximately three equal cross-sections. Score grittiness of each pickle according to the number of thirds showing grittiness and report as in (4)d. In scoring the grittiness of each section, do not include grittiness that is slight and unobjectionable to the taste. Do not section midget and gherkin size pickles but record these as whole units.
- Analysis for Grit, Sand, and Soil in Brine -- Filter brine onto paper and examine residue microscopically for presence of sand or soil particles. If appreciable amount is present, ash the filter paper and record weight of ash in mg per unit size jar.
- Report - Tabulate results as follows:
Subsample No. 1 2 etc. Totals Pickles gritty in all thirds
No.:
Percent:Pickles Pritty in two thirds
No.:
Percent:Pickles gritty in one thirds
No.:
Percent:Midgets or gherkins gritty
No.:
Percent:Grit, Sand, and Soil in brine
Wt. of Ash (mg/Jar)
(5) Procedure: Determination of Moldy Material in Pickle Relish
Stain 10 g of the well-mixed relish with a few drops of crystal violet solution [III.(3)] for 3 min. Both relish fragments and mold filaments take the stain readily. Wash out excess stain on a No. 20 sieve with water. Transfer the stained relish a small portion at a time to a petri dish and examine under water with a wide field microscope equipped with a substage light at 30-40X. To verify rotten or moldy relish fragments, check suspect fragments for mold with compound microscope if necessary, and report weight of moldy fragments. Decay caused by bacteria or yeasts will not be detected by this method.
I. Method for Pimientos (V-111)
(1) Scope
This method describes procedures for determination of rot in pimientos. The term "pimento" or "pimiento" (Capsicum annuum L.) is used rather loosely for non-pungent, fleshy varieties of red pepper. There are two distinct types of pimiento fruit:
- Oblate fruit of the tomato type
- Conically shaped fruit
Pimientos are used in salads, in cooking, and as a stuffing for green olives.
(2) Applicable Documents
(3) Defects
Pimientos in jars or cans may have decayed portions resulting from the growth of mold. These moldy areas are found more frequently on the inner surfaces of the pimiento than on the outer. They are distinguished by a brown or black discoloration. Dark-colored areas due to charring during the fire flaming process for removal of the skin may also be present, but they are easily distinguished from areas containing mold.
(4) Procedure: Determination of Rot in Whole Pimientos
- Sample Preparation -- Tare a No. 8 sieve with a light-weight drip pan. For containers of 3 lb net weight or less, use a sieve 8 in. in diameter. For containers of more than 3 lb, use a 12 in. sieve. With screen tilted about 17-20, drain contents of can or jar 2 min. Place sieve on drip pan and determine drained weight and total number of fruits. Transfer pimientos to a pan of suitable size and examine under good light.
-
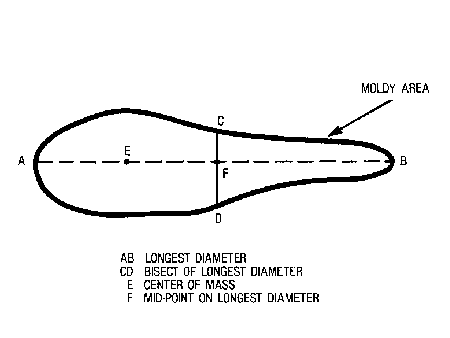
Visual Examination -- Open each pimiento and examine both inner and outer surfaces for suspected moldy areas. Confirm the presence of mold by microscopic examination of a small portion of tissue. Count and weigh the fruits containing rot. Report the size of each rotten area by averaging the lengths of the longest diameter and its bisect (Figure V-10). Dissect out decayed portions, cutting completely through the flesh, even if the rot shows on only one side. Weigh the cut-out rotten pieces. Determine and report percentage of rot by weight, using the following formula:
% rot = (wt cut-out rot/drained wt) x 100
Figure V-10
Calculation of Average Diameter of Moldy Areas
Average Diameter=(AB+CD)/2 - Report -- Tabulate results as follows:
Code No. ______ Subsample No. 1 2 3 etc. Average Pimientos per can
Drained wt (g)
No. of whole pimientosPimientos with rot
Drained wt (g)
NO.
Average diameter of rot in each piece (mm)aCut out rot
Drained wt (g)Percent rot by weight Remarks: Notes:
a If a piece contains more than one moldy area, record the average diameter of each area in the tabulation.
(5) Procedure: Determination of Rot in Pimiento Pieces (Sliced, Diced, or Chopped)
Proceed as for whole pimientos (a count of total number of pieces per can is not necessary).
J. Method for Potato Chips (V-113)
(1) Scope
This method contains a procedure for the examination of individual potato chips to determine the percent of chips that are affected by rot as demonstrated by the presence of mold filaments. Potato chips are thin slices of potato [Solanum tuberosum] fried in deep fat and then salted. Potato chips are produced in the home kitchen and on a commercial basis.
(2) Applicable Documents
(3) Defects
Many of the rots encountered in potatoes are of bacterial origin, with molds occurring as secondary rotting agents. In a number of instances, therefore, molds may not be detected in the finished rotten chip, even though the chip shows an apparently discolored, decayed area. The finding of mold in the decayed area is a definite proof of the existence of rot.
(4) Procedure: Examination for Rot in Potato Chips
- Sample Preparation and Visual Examination - Examine 8 oz. of potato chips per subsample for rot spots. Weigh subsample and count the number of chips. Rot spots are characterized by a discolored gray area. If the rot spot is large the interior may have dropped out, leaving a hole with a greyish rim. Separate the suspected rotten chips as an aliquot (a proportionate part of the subsample) for confirmation of mold. Weigh and count the number of chips in the aliquot.
- Microscopic Confirmation - Confirm the rot in the separated pieces as follows. Transfer the chips to a suitably sized beaker and add petroleum ether (at room temperature) to cover the chips and allow to remain for 15 min. Decant petroleum ether, wash with additional petroleum ether and dry. Wet a portion from the dark spot in water until soft. Mount on a microscopic slide and tease apart. Add Hertwig's solution [AOAC 44.003(v)] as a clearing agent. Warm and cover with a cover slip. Search for mold filaments at 100-200X. Calculate the weight and number of rotten chips based on the proportion of chips in the aliquot showing mold.
- Report: Tabulate results as follows:
Subsample No. 1 2 3 4 etc. Subsample Size Wt No. Rotten chips Wt No. % by wt. Remarks:
K. Method for Corn Husks (V-115)
(1) Scope
This method covers a procedure for the visual examination of corn husks to determine the percent of husks that are insect and/or mite damaged and/or affected by mildew or molds.
Dried corn husks (foliaceous bracts or spathes enclosing the ear of corn, Zea mays L.), are used as wrappers for tamales. The husks are usually imported in one of three forms:
- Unprocessed husks -- these consist of the aggregates of husks and silk, as they are taken from the field following removal of the ear.
- Partially processed husks -- these consist of individual bracts with the silk partially removed and the butt portion removed, or of naturally layered groups of a small number of bracts with the butt portion removed.
- Processed husks -- these consist of individual bracts with the worm-eaten tips and silk removed.
(2) Applicable Documents
(3) Defects
The most commonly encountered type of insect contamination is that of the corn earworm. Corn husks may contain excreta, webbing, and fragments of this insect. Spiders, spider droppings, earwigs, beetles, psocids, aphids, thrips, and mites also have been found. Occasionally corn husks are heavily mildew-stained near the tips. Other mold is usually negligible.
(4) Procedure: Determination of Reject Corn Husks
- Sample Preparation and Visual Examination -- Weigh 200 g husks from a subsample and examine all individual bracts visually for rejects due to insect infestation, mites and/or mold. Verify findings microscopically (10-30X), if necessary. The presence of mold filaments in decomposed tissue may be verified by macerating the area in water and examining at 100X. Classify according to (4)b. below and weigh each category of rejects.
-
Classification of Reject Corn Husks
-
(i) Insect-infested -- Classify as insect-infested any husk containing insects, mites, insect parts, webbing, excreta, or definite evidence of insect feeding, with the following limitations:
- Do not classify as a reject any bract containing insect-cut holes unless the tissue around one or more of the holes, over 1 mm in diameter, is obviously discolored or the bract contains a number of holes whose combined diameters exceed 1 cm (each hole measured at its greatest diameter).
- Do not classify as a reject any bract containing aphids, mites, psocids, or thrips unless these pests are so numerous that the bract is obviously unfit for its intended use.
- Do not classify as a reject any bract containing less than five insect excreta pellets without other definite evidence of insect infestation.
- (ii) Moldy -- Classify as moldy any husk or bract which contains staining mildew or other mold damage over more than 10% of its area.
-
- Report - Tabulate percent (by weight) of corn husks in each reject category and total percent rejects.
L. Method for Garlic Bulbs (V-117)
(1) Scope
This method covers a procedure for the visual examination of individual garlic bulbs to determine percent of reject bulbs due to damage by insects, molds, or other means. Garlic (Allium sativum L.) is a perennial bulbous plant. It is marketed as a compound bulb consisting of approximately 10-20 small bulbils or cloves contained within whitish membranous scales.
(2) Applicable Documents
(3) Defects
Garlic is susceptible to insect and mite infestation, and to several types of decomposition, including bulb rot (caused by Aspergillus alliaceus), blue mold rot (Penicillium spp.), and white rot (Sclerotia cepivorum).
A non-parasitic disease known as "waxy breakdown" may be due to heat, sunscald, or physiological breakdown. In the early stages, small, sunken, light-yellow areas are seen in the flesh of the clove. As breakdown progresses, these areas become deep yellow or amber throughout. The clove is usually somewhat sticky or waxy to the touch but not soft, as is the case of tissue affected by parasitic fungi. Waxy breakdown is classified in the "otherwise unfit" category.
(4) Procedure: Determination of Insect-Damaged, Moldy, and Otherwise Decomposed Garlic Bulbs
- Sample Preparation and Visual Examination -- Examine at least 50 bulbs, taking a representative number from each subsample. Examine each compound bulb separately and class it either as reject or passable. Remove the outer scales from the bulb and examine each clove by peeling and cutting as necessary. Classify the reject bulbs according to the defect definitions below. If a bulb is both insect-infested and moldy or otherwise decomposed, class it as insect-infested. Describe the decomposition.
-
Classification of Reject Bulbs
- (i) Insect-infested -- Classify as insect-infested any bulb containing live or dead insects, webbing, excreta, or definite evidence of insect feeding.
-
(ii) Moldy -- Classify as moldy any bulb containing
- Conspicuous fruiting mold or sclerotia, or
- Inconspicuous mold affecting an aggregate area larger than 1 cm2.
The presence of inconspicuous mold may be verified by magnification after identifying the affected area without magnification.
- (iii) Otherwise unfit -- Classify as otherwise unfit any bulb not classed as moldy but which is otherwise decomposed as evidenced by discoloration or other abnormal appearance affecting an aggregate area equivalent to a circle 3/4 in. or more in diameter.
- Report -- Report number and percent of bulbs in each reject category. Also report total percent rejects.
Reference
Market Diseases of Asparagus, Onions, Beans, Peas, Carrots, Celery, and Related Vegetables, Agriculture Handbook No. 303, Market Quality Research Division, USDA/ARS, Sept. 1966.