MPM: V-10. Nuts and Nut Products Methods
Macroanalytical Procedures Manual (MPM) Main Page
Contents
- Method for In-shell and Shelled Tree Nuts
- Method for Peanuts and Peanut Siftings
- Method for Microscopic Detection Of Foreign Plant Tissues in Peanut Butter
A. Method for In-shell and Shelled Tree Nuts (V-81)
(1) Scope
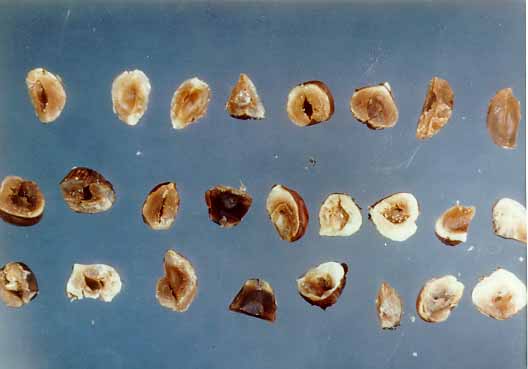
This method specifies procedures for the separation and classification of individual defective nuts or nut pieces. These are reported as percentages of reject nuts by count or weight, as appropriate, for each kind of reject. The methods are applicable, but not limited, to the following types of nuts: almonds, brazil nuts, cashews, chestnuts, filberts, pecans, pistachios, and walnuts.
(2) Applicable Documents
(3) Defects
Nuts are subject to attack by molds, insects, birds, rodents, and other vermin, which may leave harmful or obnoxious residues or otherwise damage the product material. In-shell and shelled nutmeats showing damage from such sources as well as from physiological breakdown, such as shriveling or gumminess, should be separated from clean, sound material before further processing.
Nuts of certain varieties drop free from the husk, but with other varieties, the husk must be removed by hand. Fallen nuts should be gathered several times during the harvest season as they do not all mature at the same time. Those that drop early should not be allowed to remain on the ground because they will become discolored and moldy in wet weather.
Defects characteristic of, or limited to, certain types of nuts are discussed below.
-
Almonds [Prunus dulcis (Mill.) D.A. Webb] -- Defects in almonds include brown spot, scabby blight, sculptured insect damage, and gumminess.
Brown spot is a condition caused by the "sting" (insertion of the mouth parts) of the box elder bug [Leptocoris trivittatus (Say)] before the nut is harvested. Slightly depressed brown spots, which can be removed by blanching, appear on the skin or testa of the kernel at the site of the "sting." Sculptured insect damage is a condition caused by the peach twig borer, Anarsia lineatella Zeller. The larva enters the shell of the almond in the field and remains after harvesting. It feeds on the surface of the kernel, eating away the testa in irregular or sculptured patterns. "Scabby blight" of the Jordanolo variety of almond is a hard and woody, ashen-gray or brownish scale growth on the kernel skin.
Gumminess is associated with injury from an organism such as an insect or from a mechanical source. The exact cause is not known, and the disease may vary among the different varieties of almonds. Incipient gumminess has very little effect on the edible quality of almonds, except that it gives them a sweeter taste. The condition is objectionable when the resinous glaze heavily coats a substantial portion of the kernel.
- Brazil Nuts (Bertholletia excelsa Humb. & Bonpl.) -- Brazil nuts may be attacked by mold when the pods crack and expose the nut meat to airborne molds.
- Cashew Nuts (Anacardium occidentale L.) -- Cashew nuts have a caustic liquid in the shell which protects the nut against insect attack. Shelled kernels are susceptible to attack by storage insects and rodents.
-
Chestnuts (Castanea spp.) -- The predominant commercial species is the European chestnut, C. satira Mill. Trees of the Chinese chestnut, C. mollissima Blume, bear excellent nuts and are more resistant to the fungus which causes the blight disease than are other species and hybrids. The nuts of both C. mollissima and C. satira are susceptible to spoilage by mold and decay while they are still on the tree. It is, therefore, important that the nuts be harvested promptly and regularly as they mature. Nuts may be inoculated with molds and decay organisms sometime before or at the time of maturity by certain insects that feed on them. Nuts that have become infected or that have been damaged in their cases by insects are very susceptible to spoilage. Frequent and clean collection of nuts is especially important if the nuts are likely to be infested with weevils or if the weather is hot and dry.
After harvest, chestnuts are highly perishable because of their high moisture content. Usually, 1 or 2 days of drying or curing are adequate but more time may be necessary if the air is humid. The nuts should be stored under suitable conditions of humidity (70% or less) and temperature (30 to 45C) to prevent mold growth. Rancidity, however, is usually not a problem in stored chestnuts, since the starch nuts contain very little oil.
-
Filberts (Corylus spp.) -- Filberts, also known as hazelnuts, may develop a bitter flavor due to attack by stink bugs and other plant pests. This bitter flavor is sometimes mistakenly associated with rancidity. Moldy and shriveled nuts are also serious problems. Field insects which can attack filberts are the hazelnut weevil (Curculio neocorylus Gibson) and the filbert weevil (C. occidentis (Casey)). Another field pest, the filbertworm (Melissopus latiferreanus (Walsingham)), may survive and continue development in storage.
DISCOLORATION TYPICAL OF RANCIDITY IN FILBERT KERNELS
The almond moth (Cadra cautella (Walker)) has been reported in stored filberts in California. Other storage insects may also be found in stored filberts.
-
Pecans (Carya illinoensis (Wangenh.) K. Koch) -- Pecans may develop mold at high relative humidity whether at time of harvest, storage, or processing. Certain molds directly affect the nut such as powdery mildew, which results in shriveled kernels. Leaf scorch, which may be caused by climatic stress, also results in poor filling of the kernel. Other conditions include embryo rot, pink rot, and kernel spot.
"Embryo rot" is a condition which is due to pre-harvest germination. It is characterized by dark gray to bluish-black or jet-black discoloration where the kernel halves join. The discoloration may extend the full length of the center ridge and, in severe cases, may extend into the sides on the kernel half.
"Pink rot" is caused by a mold, Cephalothecium roseum. The shell becomes oily, the kernel rancid, and as the disease progresses, the kernel is digested and replaced by a "pink powder."
"Kernel spot" is caused by the feeding of sucking insects on the immature nut. Beneath the dark surface of the "spot" the meat is white and pithy. The taste of the affected area is decidedly bitter.
- Pistachio Nuts (Pistacia vera L.) -- Pistachios stored at room temperature are subject to storage insects. In California, stored pistachios have been found to be infested with the khapra beetle (Trogoderma granarium Everts), and another dermestid beetle, T. variabile Ballion. In Greece, fungi of the genera Phomopsis and Fusarium attack the nuts secondarily through insect punctures, especially those caused by a moth larva, Tinea pistaciae Anagnos. In Iran, 25 harmful insects have been reported, including several nut-boring beetles.
-
Walnuts (Juglans spp.) -- Walnuts may turn dark-colored and become rancid. The meats may be affected by the following diseases and conditions. Bacterial blight causes black spots of various sizes on the nut meat. It also blackens the hull, shell, and kernel and, in extreme cases, renders the nut worthless. Molds cause the nut meats to be discolored and moldy. Sunburn causes the nut meats to turn dark.
Field insects which damage English walnuts include the filbertworm [Melissopus latiferreanus (Walsingham)], the navel orangeworm [Amyelois transitella (Walker)], and the codling moth [Cydia pomonella (L.)]. Codling moth damage is characterized by black-edged, darkened cavities in the kernel.
(4) Procedure: Determination of Reject In-Shell Nuts (Except Almonds)*
(* See Procedure (5) for almonds.)
- Sample Preparation -- Separate mixed nuts into individual types for separate examination. Composite 100 nuts, taking approximately equal amounts from each subsample.
- Sequential Sampling Plans -- Follow one of the sequential sampling plans given below relative to the applicable defect action level. To reach the number of nuts given in each step of the sequential plan, select nuts at random. Each subsample in the sample should contain approximately equal numbers of nuts. Examine at least 100 nuts.
10% DEFECT ACTION LEVEL Number of Nuts Examined in Subsample Number of Defective Nuts Required to: Stop Analysis Continue Analysis Stop Analysis 100 5 or less 6-14 15 or more 150 10 or less 11-18 19 or more 200 15 or less 16-23 24 or more 250 20 or less 21-28 29 or more 300 24 or less 25-33 34 or more 350 29 or less 30-38 39 or more 400 34 or less 35-43 44 or more 450 39 or less 40-48 49 or more 500 Report Results
15% DEFECT ACTION LEVEL Number of Nuts Examined in Subsample Number of Defective Nuts Required to: Stop Analysis Continue Analysis Stop Analysis 100 5 or less 6-14 15 or more 150 11 or less 12-21 22 or more 200 19 or less 20-29 30 or more 250 26 or less 27-36 37 or more 300 34 or less 35-44 45 or more 350 41 or less 42-51 52 or more 400 49 or less 50-59 60 or more 450 57 or less 58-66 67 or more 500 Report Results
- Visual and Organoleptic Examination -- Crack out the kernel, examine, and classify according to classes defined below in (4)d. Brazil nut kernels should be removed from the shell as completely as possible, cut in two or more pieces, and the cavity and all surfaces examined for mold and other evidence of decomposition. Kernels suspected of being rancid or otherwise decomposed should be tasted as necessary to confirm the condition. Separate mixed nuts into various kinds before examination and report the results for each kind separately. Report only those categories in which rejects are found. Follow the sequential sampling plan above.
-
Classification of Reject Nuts -- Following the visual and organoleptic examination, classify reject nuts according to the following categories:
-
(i) Insect-Damaged -- Classify a nut kernel as "insect-damaged" if
- It contains one or more live or dead insects, whether larva, pupa, or adult
- It shows definite evidence of insect feeding
- Insect excreta or webbing are present
State whether the insects are alive or dead, and, if possible, whether they are of field or storage origin.
Classify as insect-damaged any pecan half or large piece that shows a single or combined "spot" exceeding 1 cm in diameter. (Add the diameters of smaller spots, measuring each spot at its greatest diameter.) Classify as insect-damaged any walnut kernel having black-edged, darkened cavities caused by the codling moth.
- (ii) Moldy -- Classify a nut kernel as "moldy" if it contains any conspicuous fruiting mold, or if it contains any mold affecting more than one-fourth of its surface or an aggregate area greater than 1 cm2. The presence of mold may be verified by magnification but the area affected must be determined without magnification.
- (iii) Rancid -- Classify a nut kernel as "rancid" if it has an abnormal odor or taste characteristic of this type of decomposition. Rancid nuts frequently are soft and have a yellow, dark, or oily appearance. Classify as rancid any pecan kernels showing pink rot [see (3)f].
- (iv) Otherwise Decomposed -- Place in this category any nut kernel that is not classified as moldy or rancid, but is otherwise decomposed as evidenced by discoloration or other abnormal appearance or flavor so that it is unfit for food. Describe the decomposition. Classify as "otherwise decomposed due to embryo rot" any pecan kernel which has dark discoloration extending more than two-thirds of the length of the center ridge or an equivalent amount in other portions of the kernel.
- (v) Dirty -- Classify a nut kernel as "dirty" if the surface is heavily smeared, thickly flecked or coated with dirt, seriously affecting its appearance.
- (vi) Blank -- An unshelled nut is a "blank" if the kernel is so shrunken or improperly matured that it is inedible or worthless. Count each blank as a full reject.
- (vii) Shriveled -- An unshelled nut is "shriveled" if the kernel is less than one-half of its apparent normal size. Count each shriveled nut as a full reject.
-
- Report -- Tabulate results from sequential plans as follows:
Subsample No. 1 2 3 etc. Average No. Examined No. Insect-Infested No. Moldy No. Rancid No. Otherwise Decomposed No. Blanks No. Shriveled No. Dirty Total No. Rejects Total % Rejects
(5) Procedure: Determination of Reject Shelled Nuts (Whole, Half Kernels, Large Pieces) and In-Shell Almonds
- Sample Preparation -- Prepare samples as described in (4)a.
- Sequential Sampling Plan -- Follow the sequential sampling plan below. To reach the number of nuts given in each step of the sequential plan, select nuts at random. Each subsample in the sample should contain approximately equal numbers of nuts. Examine at least 100 nuts.
5% DEFECT ACTION LEVEL Number of Nuts Examined in Subsample Number of Defective Nuts Required to: Stop Analysis Continue Analysis Stop Analysis 100 1 or less 2-8 9 or more 150 3 or less 4-10 11 or more 200 6 or less 7-12 13 or more 250 8 or less 9-15 16 or more 300 10 or less 11-17 18 or more 350 13 or less 14-19 20 or more 400 15 or less 16-22 23 or more 450 17 or less 18-24 25 or more 500 Report Results
- Visual and Organoleptic Examination -- Examine shelled nuts, using the naked eye or up to 10X magnification. Separate mixed nuts into various kinds before examination and report the results for each kind separately. Classify the rejected nuts according to the classes defined in (5)d.
-
Classification of Reject Nuts -- Following the visual and organoleptic examination, classify reject nuts in the following categories:
- (i) Insect-Damaged -- See (4)d.(i). Count as one-half of an insect-damaged reject any almond kernel showing an area or aggregate area of brown spot greater than the area of a circle 1/8 in. in diameter. Classify as insect-damaged any almond showing sculptured insect damage by the peach twig borer affecting 10% or more of the surface.
- (ii) Moldy -- See (4)d.(ii)
- (iii) Rancid -- See (4)d.(iii)
- (iv) Otherwise Decomposed -- See (4)d.(iv). Classify as "otherwise decomposed due to scabby blight" any almond kernel which shows a single or combined scabby area exceeding 13 mm. Add the diameters of smaller areas (each measured at its greatest diameter)
- (v) Dirty -- See (4)d.(v)
- (vi) Shriveled -- See (4)d.(vii)
- (vii) Gumminess -- This is a condition in almonds characterized by a resinous glaze which coats the kernel. Count a gummy almond as a full reject if the resinous glaze heavily coats more than one-half the kernel surface.
- Report. Report the results using the table provided in (4)e. Report the number of rejects in each category and the total number and percentage of rejects. List only those categories in which rejects are found. Add a category for "Gumminess" if necessary.
References
(1) Gecan, J.S., P.M. Brickey, Jr. and W.V. Eisenberg, "Insect Problems of Pecan Shelling Plants and Their Relation to Insects and Insect Parts in Processed Pecans," J. Food Sci. 36: 89-92, 1971.
(2) Nut Tree Culture in North America, Ed. by Richard A. Jaynes, Northern Nut Growers Assoc., Hamden, CT, 1979.
(3) Woodruff, J.G., Tree Nuts Production, Processing & Products, Vols. I and II, AVI Publishing Co., Inc., Westport CT, 1967.
B. Method for Peanuts and Peanut Siftings (V-89)
(1) Scope
This method describes the following procedures:
- Examination of individual peanuts to determine the percent of rejects due to damage by insects, molds, rancidity, decay and other adhering material
- Screening of whole bags of shelled peanuts to determine the presence of insects or other extraneous material.
(2) Applicable Documents
- CPG 7112.04 Defect Action Level
- I0M 429.1 Whole-Bag Screening
(3) Defects
The concern about aflatoxins and the research on the causative mold, Aspergillus flavus, in peanuts has resulted in great improvements in prevention of damage to peanuts from molds, insects, and other causes during harvest, shelling, and storage. The industry maintains strict controls to eliminate defective peanuts since experience has shown that aflatoxin is most frequently associated with moldy, discolored, shriveled, insect-damaged, or otherwise damaged peanuts.
- Insect Infestation and Damage -- Harvested split or broken pods are susceptible to infestations by insects and molds during the curing process. After shelling, the kernels are highly susceptible to stored product insect infestations. Storage conditions which protect against infestation, such as cold storage, and inspection and fumigation are needed.
- Moldiness, Rancidity, and Decay -- Peanut pods are seldom attacked by molds in the field except during adverse weather and growing conditions. After being dug, pods are cured (dried) as rapidly as possible to a safe moisture level to prevent growth of molds or other microorganisms. This is important, as molds may develop on moist kernels when temperature changes cause "sweating" or condensation. Rancidity or decay may also affect the kernels. In the earlier stages of rancidity the kernel may have only a slightly abnormal appearance in the form of an oily or somewhat translucent condition. This condition is frequently found following freezing injury. In most cases, rancid and decayed kernels that are decidedly off flavor are easily detected by their appearance.
-
Adhering Material on Peanut Kernels
Figure V-7
ADHERING MATERIAL ON PEANUT KERNELSLine 1: Not Scorable
Whole or half kernels with adhering material equal to or less than the amount shown are not scored.Line 2: Minor Defects.
Whole or half kernels with adhering material in equal or greater amounts than shown are scored as minor defects.Line 3: Damage.
Whole or half kernels caked or badly smeared with material are scored as damage when occuring in equal or greater amounts than illustrated. Ground-in material or gouged surfaces which are dirty are more objectionable and lesser amounts are scored as damaged.-- Normal milling and handling of shelled peanuts can result in accumulation of peanut meal, skin particles or small quantities of dirt on the kernel surface where the skin is missing (see Figure V-7). This adhering material affects the appearance of the kernels whether it only lightly coats the kernel or has been ground into the surface. Kernels with ground in material or gouged-surfaces which appear dirty are clearly more objectionable than kernels with lightly caked material on their surfaces.
(4) Procedure: Determination of Rejects in Unshelled Peanuts
Follow 10.A.(4).
(5) Procedure: Determination of Rejects in Shelled Peanuts
- Sample Preparation and Visual Examination -- Weigh a representative portion of 100 g from each subsample. Examine each kernel under good lighting. Peel, split, or cut, as necessary, to determine the extent of any damage and classify reject nuts according to the applicable categories in 10.A.(4)d. Do not use the sequential sampling plan.
- Report - Tabulate results as follows:
Subsample No. 1 2 3 etc. No. examined (g) No. Insect-infested (g) No. Moldy (g) No. rancid (g) No. otherwise decomposed (g) No. dirty due to adhering material (g) No. total rejects (g) Percent total rejects Remarks:
(6) Procedure: Determination of Extraneous Material in Siftings from Whole Bags of Shelled Peanuts
- Sample Preparation -- Subsamples as submitted will usually consist of siftings from previously screened large containers of peanuts (i.e., 50-125 lb bags). If a container of peanuts is submitted, pass a small portion at a time over a large 1/4 in. mesh shelled peanut sieve*. Collect the siftings.
- Visual Examination -- Weigh and examine the siftings in good lighting with the naked eye, or with such magnification as necessary, to confirm findings of whole insects or equivalent, rodent excreta pellets, and other extraneous material. Note presence of any live insects. If the magnification exceeds 10X this should be stated in the report of results.
-
Report -- Tabulate results for each subsample according to applicable microscopic categories, using the format of AOAC 970.66B(i).
* The sieve is made by framing a 2 x 4 ft piece of hot dip, heavy duty galvanized screen with 1/4 x 1/4 in. openings and 1/16 in. diameter wire. The screen is stapled to the wooden frame and held with molding. The over-all dimensions of the frame are 44-3/8 x 24-1/2 in. It is made from 1-3/8 x 2-5/8 in. dressed lumber.
C. Method for Microscopic Detection Of Foreign Plant Tissues in Peanut Butter (V-92)
(1) Scope
This method provides a procedure for detection of foreign plant tissues in peanut butter and peanut butter spreads.
Peanut butter is a paste made from grinding shelled and skinned roasted peanuts. Some peanut butter "spreads" may also contain soy or wheat flour.
(2) Applicable Documents
(3) Defects
Some peanut butter manufacturers may add undeclared foreign plant substances to their products as cheaper substitutes without declaring such on the label.
(4) Procedure: Examination for Foreign Plant Tissues
Defat 100 g peanut butter with three 150 mL portions of petroleum ether for 30 min each. Filter defatted tissues on ruled paper. Make up at least three microscope slides from the defatted peanut butter, one with water and two or more with chloral hydrate solution. Examine the water mount microscopically for foreign starch grains at 100-200X. Draw in a drop of iodine/potassium iodide solution to stain the grains by placing a piece of filter paper on the opposite side of the slide. If foreign starch grains are detected, identify them by using determinative starch keys and/or by comparison with slides of authentic material. Examine the other slides for presence of any foreign plant tissues and if detected, identify in a similar manner.
If the results of microscopic examination of the defatted peanut butter are not conclusive, digest 2-3 g of the defatted peanut butter according to the AOAC method for crude fiber. The digestion will concentrate and clear the tissues for microscopic examination. Sclerenchyma and lignified tissues from foreign food seeds are more readily detected with this process. Examine several representative slides at 100-400X to determine whether any foreign plant tissues are present. If detected, identify by comparison with authentic material.
Report any foreign plant tissues as an apparent undeclared ingredient. Identify if possible. Estimate percent of foreign tissues by microscopic comparison with peanut butter containing known percentages of added authentic material.