Laboratory Information Bulletin (LIB) 4363: Malachite Green and Leucomalachite Green in Fish and Shrimp
Quantitative and Confirmatory Analyses of Malachite Green and Leucomalachite Green Residues in Fish and Shrimp
This document is also available in PDF (414 Kb).
Volume 21, No. 11, November 2005
Wendy C. Andersen, Sherri B. Turnipseed, and José E. Roybal
U.S. Food and Drug Administration, Animal Drugs Research Center, Denver, CO
Note: The Laboratory Information Bulletin is a communication from the Division of Field Science, Office of Regulatory Affairs, U.S. Food and Drug Administration for the rapid dissemination of laboratory methods (or scientific regulatory information) which appear to solve a problem or improve an existing problem. In many cases, however, the report may not represent completed analytical work. The reader must assure, by appropriate validation procedures, that the reported methods or techniques are reliable and accurate for use as a regulatory method. Reference to any commercial materials, equipment, or process does not, in any way, constitute approval, endorsement, or recommendation by the U.S. Food and Drug Administration.
ABSTRACT
Liquid chromatographic methods are presented for the quantitative and confirmatory determination of malachite green (MG) and leucomalachite green (LMG) in catfish, trout, tilapia, basa, salmon, and shrimp. Residues were extracted from tissues with ammonium acetate buffer and acetonitrile, and isolated by partitioning into dichloromethane. Leucomalachite green was quantitatively oxidized to the chromic malachite green by reaction with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. Samples were cleaned-up by solid phase extraction with alumina and propylsulfonic acid phases. Extracts were analyzed for total MG by liquid chromatography with visible detection (LC-VIS) at 618 nm, using isocratic elution and a C18 column. The method was validated in each species fortified with LMG at 1, 2, 4, and 10 ng/g (ppb). Average recoveries were 85.9 % (± 8.5 % RSD) for channel catfish, 87.8 % (± 5.1 % RSD) for rainbow trout, 93.9 % (± 8.9 % RSD) for tilapia, 90.8 % (± 11.0 % RSD) for basa, and 89.9 % (± 8.4 % RSD) for tiger shrimp. A slight variation to this extraction procedure was previously validated for LMG and MG in salmon;1,2 this report contains additional salmon data showing that the two extraction procedures produce equivalent results for salmon analysis. MG was confirmed in fish extracts by ion trap LC-mass spectrometry (LC-MSn) with no discharge-atmospheric pressure chemical ionization (ND-APCI). In addition to providing confirmatory data, the LC-MSn method provides an alternative quantitative method for MG in fish. The recoveries of LMG, measured as MG by this LC-MSn method, at all fortification levels (1-10 ppb) were high (75-116 %) with relative standard deviations of 21 % or less. The results agreed very closely with those obtained from the same extracts using the LC-VIS analysis procedure, indicating that matrix suppression was not an issue with LC-MSn. This study also included the determination of MG and LMG residues in tissues from catfish, trout, tilapia, and salmon that had been treated with MG. Malachite green was quantitatively determined at the LC-VIS method detection limit of 1.0 ppb; detection limits of approximately 0.25 ppb are possible with LC-MSn.
INTRODUCTION
Malachite green (MG) is an effective topical fungicide used in the aquaculture industry. MG absorbed by fish tissue is metabolically reduced to leucomalachite green (LMG), which is lipophilic and can be stored in edible fish tissues for an extended period of time.3 It is, therefore, likely that the majority of violative residues present in fish will be in the form of LMG.
Due to the suspected mutagenicity of MG and LMG,4-6 MG is not permitted for use as an aquaculture veterinary drug in many countries including the United States, Canadaand in the European Union. Regardless, numerous reports of MG misuse in aquaculture have been reported in the US and internationally.7 The European Commission requires that methods be able to determine the sum of MG and LMG residues at the minimum performance limit of 2 ng/g.8
MG is positively charged and has a strong chromophore at 618 nm, making it amenable to both LC with visible absorbance detection and no-discharge APCI LC-MSn.2 Because the residue is found primarily as the reduced leuco compound in fish tissue, oxidation is required to convert this residue to the colored, charged form of the drug. In this report, we present a quantitative LC-visible method and confirmatory LC-MSn method for the analysis of LMG and MG incorporating an in-situ DDQ oxidation.1 The method does not distinguish between LMG and MG residues that may be present, but rather allows quantification of the sum of the residues, both of which are prohibited in edible fish tissues. The LC-MSn method can provide complementary quantitative data. The method has been validated in catfish, trout, tilapia, basa, and shrimp, and verified in other species. A slight variation to this extraction procedure was previously validated for LMG and MG in salmon.1,2,9,10 This report contains additional salmon data showing that the two extraction procedures produce equivalent results for salmon analysis.
EXPERIMENTAL
Equipment and reagent sources have been provided for information and guidance. Equivalent products may be substituted as appropriate.
Equipment
- Liquid chromatograph - Agilent model HP1100 series II with programmable diode array detector (DAD) (Avondale, PA). Operating conditions: 1.0 mL/min mobile phase flow rate; 35°C column temperature; 65-140 bar column pressure; 100 µL volume injected. DAD was set at an absorbance wavelength of 618 nm (4.0 nm bandwidth) with a reference of 725 nm (8 nm bandwidth) using a tungsten lamp.
- Liquid chromatograph mass spectrometer - Agilent (Avondale, PA) 1100 LC interfaced to a ThermoElectron (San Jose, CA) Finnigan DECA-XP Plus Ion Trap MS with an atmospheric pressure chemical ionization (APCI) source. XCaliber (Version 1.3) was the software used to operate both instruments.
- LC column - Alltech Alltima C18, 3 µm, 150 x 4.6 mm id. (P/N 81385), with guard cartridge (5 µm, 7.5 x 4.6 mm, P/N 96080) of the same phase (Alltech Associates, Deerfield, IL), and column pre-filter (ColumnSaver, 0.5 µm (P/N MMCS-210), MacMod Analytical Inc., Chadds Ford, PA).
- LC-MS column - YMC phenyl 3-4-5 cartridge column, 3 µm, 120 Å, 4.0 x 50 mm, (P/N PH12S030504WTA) (Waters Corp., Milford, MA), with a guard cartridge insert (4.0 x 20 mm) of the same phase.
- Blender/homogenizer -RobotCoupe Blixer, homogenizer, 4 quart, model RS1BX4V (RobotCoupe USA, Inc., Ridgeland, MS).
- Vortex Mixer - Vortex Genie 2, (Scientific Industries, Bohemia, NY).
- Centrifuge - refrigerated to 0°C, capable of accelerating 50 mL tubes to 4000 rpm.
- Rotary evaporator - Büchi model R-110 with ice trap, evaporation temperature 50°C (Brinkmann Instruments, Inc. Westbury, NY).
- Alumina (ALN-SPE) columns - Bakerbond alumina solid phase extraction columns, neutral, 6 mL, 1000 mg, disposable, P/N 7214-07 (JT Baker Inc., Phillipsburg, NJ).
- Propylsulfonic acid (PRS-SPE) columns - Bond Elut LRC propylsulfonic acid solid phase extraction columns, 500 mg, disposable, P/N 1211-3038, with LRC 10 mL column connection adaptors, P/N 1213-1003 (Varian Inc., Palo Alto, CA).
- SPE manifold - commercial SPE vacuum elution manifold with water aspirator and vacuum gauge.
- Centrifuge tubes. - 50 mL disposable, conical, graduated, polypropylene tubes with cap; 15 mL disposable, conical, graduated, polypropylene tubes with cap.
- Volumetric glassware and pipettes - 100.0 and 50.0 mL volumetric flasks, class A; 15 mL graduated centrifuge tubes with glass stoppers 13; adjustable volume pipettors with disposable polypropylene tips - 10-100 µL (Eppendorf, Brinkmann Instruments, Inc., Westbury, NY), 200-1000 µL (Ulster Scientific, Inc., New Paltz, NY), and 1-5 mL (Wheaton Science Products, Millville, NJ).
- Glassware - 150 mL pear-shaped boiling flasks with 24/40 necks and glass stoppers; 250 mL separatory funnels with PTFE stopcocks and glass stoppers 22; 5.75 or 9 inch disposable Pasteur pipettes; clear or amber glass LC vials, 2 mL volume, with crimp, snap, or screw caps.
Reagents
- Reference standards - malachite green (MG), (CI 42000) as the oxalate salt, FW = 929.0 (CAS 2437-29-8), (M-6880, Sigma, St. Louis, MO); leucomalachite green, (LMG) FW=330.48, (CAS 129-73-7) (12,5660, Aldrich, St. Louis, MO).
- Solvents - high purity chromatographic and spectrophotometric grade acetonitrile and methanol were used. Dichloromethane was liquid chromatographic grade. All water used was deionized and purified to 18.2 MΩ·cm (Millipore, Bedford, MA).
- Acetic acid - glacial, ACS grade, aldehyde-free, (CAS 64-19-7).
- Ammonium acetate - anhydrous, 98 % purity, FW = 77.08 (CAS 631-61-8).
- 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone, (DDQ) - 98 % purity, FW = 227.01 (CAS 84-58-2).
- 0.01 M DDQ stock solution: weigh 0.227 g DDQ into 100 mL volumetric flask and dilute to volume with acetonitrile; solution may be stored tightly capped and stored in the refrigerator for up to one month.
- 0.001 M DDQ working solution: pipette 5 mL of the 0.01 M DDQ solution into a 50 mL volumetric flask and dilute to 50 mL with acetonitrile; this solution was stored at room temperature and prepared fresh weekly.
- Diethylene glycol (DEG) - reagent grade (CAS 111-46-6).
- Alumina - chromatographic grade, 80-200 mesh (CAS 1344-28-1) (AX0612-1, EM Science, Gibbstown, NJ).
- Hydroxylamine hydrochloride (HAH) - ACS reagent grade (99.8%), crystalline, FW = 69.49 (CAS 5470-11-1); 0.25 g/mL solution: weigh 25.0 g HAH into a 100 mL volumetric flask and dilute to mark with DI water.
- p-Toluene sulfonic acid (p-TSA) - 98.5 %, monohydrate, FW 190.22 (CAS 6192-52-5); 1 M solution: weigh 9.5 g p-TSA into a 50 mL volumetric flask and dilute to mark with DI water.
- Formic Acid - ACS grade, 96 %, (CAS 64-18-6); 0.1 % solution: pipette 1 mL into a 1000 mL graduated cylinder and dilute to mark with DI water.
- Ammonium acetate buffer - Prepare a 0.1 M ammonium acetate solution by dissolving 7.7 g ammonium acetate in a 1000 mL DI water. Adjust 1 L of this solution to pH 4.5 by adding 8 mL of acetic acid and 5 mL of 1 M p-TSA (equivalent to 5 mM p-TSA). Use this buffer to prepare mobile phase A, as described in (l).
- Mobile phase - Mobile phase A is a 1:1 mixture (by volume) of ammonium acetate buffer and acetonitrile (filtered through a 0.45 µm PVDF membrane before use). Mobile phase B is acetonitrile. The LC isocratic mobile phase elution profile is 95 % A and 5 % B (equivalent to 47.5 % ammonium acetate buffer and 52.5 % acetonitrile). Mobile phase A is also used in this method to dilute calibration standards and elute SPE columns.
Standard Preparation
- Stock Solutions: 100 µg/mL. Accurately weigh 10.0 mg of each reference standard (corrected for MG and LMG purity) into separate 100 mL volumetric flasks (for LMG use low-actinic glass or wrap volumetric with aluminum foil to protect from light), dilute to volume with methanol and mix. Note: the mass of MG was also corrected for the MG-oxalate product, which contains two molecules of MG for one molecule of the MG-oxalate dimer complex (there are 7.109 mg of MG per 10 mg of MG-oxalate dimer). (MG-ICV): A second MG stock solution should be prepared as the Initial Calibration Verification (ICV). Stock solutions should be stored at room temperature, protected from light, and freshly prepared every six months.
- MG Standard Solutions for Calibration Curve Only:
(MG1, 1.0 µg/mL): Pipette 1.0 mL of the MG stock solution into a 100 mL volumetric flask, dilute to volume with methanol and mix. (MG1-ICV, 1.0 µg/mL): Prepare a second 1.0 µg/mL MG working standard from the MG-ICV stock solution. MG1 was freshly prepared monthly and stored at room temperature.
(MG2, 0.1 µg/mL): Pipette 1.0 mL of (MG1) into a 15 mL graduated glass centrifuge tube and dilute to 10 mL with methanol. (MG2-ICV, 0.1 µg/mL): Prepare a second 0.1 µg/mL MG working standard from the MG1-ICV working standard solution. MG2 was freshly prepared weekly and stored at room temperature.
Prepare a series of LC calibrants by aliquoting into individual 15 mL graduated glass or disposable polypropylene centrifuge tubes, 50 µL, 100 µL, and 200 µL of the 0.1 µg/mL MG2 solution and 50 µL and 100 µL of the 1.0 µg/mL MG1 solution. Dilute each to 5.0 mL with (1:1 vol) acetonitrile/ammonium acetate buffer (mobile phase A), vortex mix or stopper and invert to mix thoroughly. Based on a 5 g sample weight, these solutions will generate standards with concentrations of 1, 2, 4, 10 and 20 ng/g of MG. Prepare a 4 ng/g ICV standard by diluting 200 µL of the 0.1 µg/mL MG2-ICV solution to 5.0 mL with mobile phase A. Calibration standards were prepared every 1 to 2 days. Consistent use of either glass or polypropylene tubes should be adopted to avoid slight volume differences between LC calibrants and final sample extracts.
-
LMG Standard Solutions for Sample Fortification Only:
(LMG1, 1.0 µg/mL): Pipette 1.0 mL of the LMG stock solution into a 100 mL low-actinic volumetric flask, dilute to volume with methanol and mix. LMG1 was freshly prepared monthly and stored at room temperature.
(LMG2, 0.1 µg/mL): Pipette 1.0 mL of LMG1 into a 15 mL graduated glass centrifuge tube and dilute to 10 mL with methanol. LMG2 was freshly prepared weekly and stored protected from light at room temperature.
Sample Preparation
Thawed fish fillets were cut into 3 to 5 cm cubes and placed in a freezer (-20 to -30°C) until use. Trout and salmon tissue was processed with skin intact (scales removed). Shells were removed from shrimp before processing. Frozen fish or shrimp samples were blended with dry ice in a blender/homogenizer with pulsed action until contents were uniform and had the consistency of a fine powder. Homogenate was placed in Whirl-Pak bags, loosely sealed and stored in the freezer overnight to allow the carbon dioxide to dissipate. Whirl-Pak bags were then tightly sealed until analysis. In this study, aquacultured fillets of fresh rainbow trout, tilapia, and Atlantic salmon, and imported frozen basa fillets and headless tiger shrimp were purchased at a local market. Frozen channel catfish fillets were provided from the FDA Gulf Coast Seafood Laboratory.
Sample Fortification
To generate validation data, 5.0 g portions of thawed tissue composite were fortified by spiking with 50, 100, or 200 µL of LMG2 or 50 µL of LMG1 solutions to produce samples containing 1, 2, 4 or 10 ng/g of LMG, respectively. Samples fortified with MG at 2 ng/g were generated by spiking 5.0 g of tissue with 100 µL of the MG2 solution. Samples were allowed to sit at room temperature for at least 15 minutes before proceeding with extraction.
Incurred Tissues
One of each catfish, trout, tilapia, and salmon fish were placed into individual water baths containing 10 µg/L (ppb) MG for 1 hour. The fish were then returned to clean water, and sampled after depuration times of 16 - 24 hours after MG exposure. One of each species of unexposed control fish was also sampled. Fish were filleted (skin was left intact on the trout and salmon), frozen, and blended with dry ice according to sample preparation method above.
Extraction
Accurately weigh 5.0 g tissue composite into a 50 mL centrifuge tube and let thaw. Add 5 mL of ammonium acetate buffer, 1 mL of HAH solution and 100 µL of p-TSA solution. Cap the centrifuge tube and mix by vortexing vigorously for 30 seconds. Add 25 mL of acetonitrile, cap and shake vigorously for 30 seconds. Add 10 g of alumina, cap and shake vigorously for 15 seconds. Centrifuge at 0°C for 5 minutes at 4000 rpm (2730 rcf).
Add 50 mL water and 2 mL DEG to a 250 mL separatory funnel. Decant the sample supernatant into the separatory funnel. Add another 25 mL of acetonitrile to the solids remaining in the 50 mL centrifuge tube, cap, mix by vortexing for 30 seconds, and then shake vigorously for 30 seconds. Centrifuge at 0°C for 5 minutes at 4000 rpm. Decant supernatant into original 250 mL separatory funnel containing first extract. Add 25 mL dichloromethane to separatory funnel, stopper, invert and open stopcock to release pressure. Close stopcock and extract by mixing funnel in bicycle motion for 30 seconds. Allow phases to separate for no more than 10 minutes in the case of catfish, trout, tilapia, basa, and shrimp (salmon may require up to 15 minutes to separate1). Drain the lower dichloromethanelayer into a 150 mL pear-shaped boiling flask, collecting the last few drops by touching the tip of the separatory funnel to the boiling flask. Add an additional 25 mL of dichloromethaneto the 250 mL separatory funnel, and repeat the liquid extraction as before. Let the phases separate for 10 minutes, then collect the organic phase in the original 150 mL boiling flask, combining the first and second extracts.
Rotoevaporate the contents of the boiling flask to dryness under reduced pressure while heating flask in a water bath set at 50°C. Add 3 mL of acetonitrile to the dried oily residue and swirl to dissolve the residue. At this point, sample may be stoppered and stored overnight at room temperature and protected from light. Add 3 mL of 0.001 M DDQ solution and swirl to mix. Sample will immediately change from orange to a dark red-purple color. Allow oxidation reaction to proceed for 30 minutes, with periodic sample agitation. The color of the sample may lighten over time; however, if the sample pales significantly or clears, a lower than average recovery may be obtained.1
While the oxidation reaction is occurring, condition disposable ALN-SPE and PRS-SPE columns with 5 mL of methanol followed by 5 mL of acetonitrile. Add an additional 5 mL of acetonitrile to the PRS-SPE to serve as a solvent reservoir. Position the ALN-SPE column above the PRS-SPE column using a column adapter and insert the SPE in a vacuum elution system. Transfer the oxidized sample solution to the ALN-SPE, and adjust vacuum to allow sample to elute onto the PRS-SPE column at a drop rate of approximately 4 mL/min. Wash boiling flask with two sequential portions of 5 mL acetonitrile and add each wash to the ALN-SPE just as the previous liquid portion clears the upper column. Remove the ALN-SPE column and discard. Wash the PRS-SPE with 5 mL acetonitrile. Pull vacuum on the PRS-SPE column for 2-3 seconds to remove most of the residual acetonitrile (column should not be fully dried). Remove PRS-SPE from the vacuum elution system and elute by gravity into 15 mL graduated centrifuge tube with 4 mL of mobile phase A (1:1 ammonium acetate buffer:acetonitrile). With hand bulb or syringe, blow out remaining solvent from column into centrifuge tube. Dilute sample to 5.0 mL with mobile phase A, and transfer a portion of the sample to a chromatographic vial for LC-visible analysis. The remaining portion of this sample may be transferred to a second chromatographic vial for simultaneous confirmation by ND-APCI LC-MSn or stored in the refrigerator (ca. 4°C) for several days for later residue confirmation. Saved samples should not be frozen.
Quantification by LC-VIS
The LC was operated with a mobile phase flow rate of 1.0 mL/min and column temperature of 35°C. All injections were 100 µL and a needle wash of Mobile Phase A was used. The DAD was set at an absorbance wavelength of 618 nm (4.0 nm bandwidth) with a reference of 725 nm (8 nm bandwidth) using a tungsten lamp. A calibration curve was generated daily from the peak area response of six MG standards with concentrations 0, 1, 2, 4, 10, and 20 ng/mL. Injection of the 4 ng/g calibration standard gave an approximate peak area response for MG of 4.6 mAU·s (0.21 mAU peak height). During 8 months of LC column use, the MG retention time ranged from 8.9 to 11.7 minutes, with an average retention time of 10.3 minutes. Correlation coefficients (r2) for acceptable standard curves were at least 0.995.
As discussed below, although the fortified samples are spiked with LMG, the DDQ oxidation quantitatively converts all of the LMG in the sample to MG. Recovery of LMG is therefore determined from the total residue as MG, which is calculated from the MG calibration curve. After injecting all of the calibration standards and samples, the 2 or 4 ppb standard should be reinjected as the Continuous Calibration Verification (CCV). The 4 ppb ICV standard should be within ± 10 % of the peak area counts of the 4 ppb standard and CCV should be within ± 10 % of the peak area counts of the initial injection of that standard. After the injection of each set of standards and samples, or at the end of each day, the chromatography column should be flushed with 100 % methanol for 30 minutes.
Residue Confirmation and Quantification by ND-APCI LC-MSn
The LC-MS was tuned by flowing MG standard solution (0.5 ng/µL MG in 50/50 water/methanol) at a rate of 10 µL/min using a syringe pump into a stream of 700 µL/min 63/37 0.1% formic/ACN via a T fitting. Source parameters such as lens voltages, gas flows, vaporizer and capillary temperatures, and collision energies were optimized in this manner. Typical MS parameters for no-discharge APCI were determined to be: corona discharge 0 µA; vaporizer temperature, 400 °C; capillary temperature, 220°C; capillary voltage, 40V; sheath gas, 70; auxiliary gas, 40. The number of prescans was equal to two and the maximum inject time was set to 500 ms for MS2 scans. The MS acquisition programs consisted of a MS2 scan of m/z 329 with isolation width of 2 amu, collision energy = 48 or 50, Q = 0.25, activation time = 30 ms, and range = m/z 150-350.
The final instrument procedure consisted of a 15 minute LC program that was isocratic (63/37 0.1% formic/ACN) for the first 10 minutes, followed by a quick gradient to 100% ACN from 10 to 10.5 minutes, a column wash of 100% ACN from 10.5-12 minutes, a ramp back to 63/37 0.1% formic/ACN from 12 to 12.5 minutes, and equilibration at that composition for the final 2.5 min. The column oven was maintained at 30 °C. The mobile phase flow was 700 µL/min. Ten microliter injections were made with a needle wash of water or methanol. The divert valve was switched to the MS at 1 min and to waste at 9.8 min.
The treatment of the data varied depending on whether qualitative (confirmatory) or quantitative data were being evaluated. For qualitative assessment, individual ion transition chromatograms (m/z 329, 313-315, 284-286, 251, and 208) were generated and the resulting chromatographic peaks were integrated with the ICIS algorithm. A Gaussian smoothing function of five points was applied. Relative abundances were calculated from these peak areas and compared to contemporary standards. For quantitative assessment the area counts of the MG peak from the total ion chromatogram of the m/z 329 product ion scan, not the extracted ion chromatograms, were used. Peak areas were calculated by the Quan Browser® software program and the ICIS integration program was selected for the processing method. A calibration curve was generated with the same standards as were used for the LC-VIS analyses. When the calculated amount of MG was less than 1 ng/g, the 20 ng/g standard was excluded and the data was reprocessed with Quan Browser®.
Results and Discussion
The extraction method presented herein is a modification of one recently validated for LMG and MG residue determination in salmon.1 Two changes were made to the original salmon method to ensure high residue recovery of LMG and MG in catfish. Most importantly, the quantity of alumina used in the initial extraction was increased from 6 g to 10 g. When 6 g of alumina was used for the extraction of LMG residues in catfish, recoveries of ten 4 ng/g spikes ranged from only 33 to 59 %. Recoveries were dramatically higher when 10 g of alumina was used in the extraction as shown in Table 1. Secondly, during the liquid-liquid extraction step, the organic and aqueous phases should not be in contact for more than 10 minutes. Although the original salmon method called for a phase separation time of 15 minutes, lower recoveries were again observed for catfish with long separation times. Catfish, trout, tilapia, basa, and shrimp samples all separated within a few minutes during the liquid-liquid extraction, making separation times longer than 10 minutes unnecessary. In this study, fifteen fortified salmon samples were extracted using 10 g of alumina with an average recovery of 92.7 % (10.0 % RSD) (Table 1). These results are comparable to the 95.4 % average recovery (11.1 % RSD, n = 35) obtained from the previous salmon study.1 In addition, ten incurred salmon samples were extracted using either 6 g or 10 g of alumina. The five samples extracted with 6 g of alumina had an average combined MG/LMG concentration of 29.7 ng/g (6.4 % RSD), as compared with the five 10 g alumina samples, which had an average combined MG/LMG concentration of 26.4 ng/g (3.2 % RSD). These results indicate that the current method can be used for the analysis of catfish, trout, tilapia, basa, shrimp, and salmon. Moreover, salmon extracted with the increased quantity of alumina did not have the previously seen problems either with emulsions (organic and aqueous phases separated within 10 minutes) or with DDQ oxidation.1
The method requires approximately 5 hours to extract 6-10 samples and prepare calibration standards. The solvent evaporation step is the most time consuming, requiring approximately 15 to 20 minutes to evaporate each sample. Greater sample throughput is possible with additional rotary evaporation units. In addition to catfish, trout, tilapia, basa, salmon, and shrimp, the method has also been verified for use for LMG in eel and broadhead clarias fish.
Residue Quantification by LC-VIS
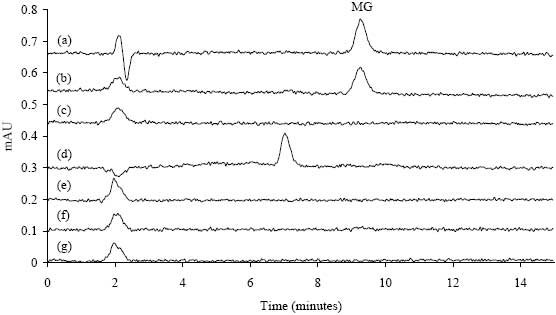
The average recoveries of MG from catfish, trout, tilapia, basa, salmon, and shrimp fortified with LMG at 1, 2, 4, and 10 ng/g are shown in Table 1. Overall recoveries were 85.9 % (± 8.5 % RSD, 47 samples) for catfish, 87.8 % (± 5.1 % RSD, 32 samples) for rainbow trout, 93.9 % (± 8.9 % RSD, 37 samples) for tilapia, 90.8 % (± 11.0 % RSD, 21 samples) for basa, 92.7 % (10.0 % RSD, 15 samples) for salmon, and 89.9 % (± 8.4 % RSD, 33 samples) for shrimp. Typical chromatograms are shown in Figure 1 for a 2 ng/g MG standard, catfish fortified with 2 ng/g LMG, and control fish. The aquacultured salmon and trout samples typically had some background peaks in the chromatogram,1 catfish, tilapia, basa, and shrimp were free from interference.
Malachite green was not detected in any of two reagent blanks, nine control tilapia samples, eight control shrimp samples, or four control salmon samples. An interference peak was found in one of eleven control catfish samples and in one of eight control trout samples, but the interference peaks were too small to be quantified by the LC-VIS method. As discussed in greater detail below, MG was also confirmed in the control catfish sample LC-MSn, although the concentration was less than 0.25 ng/g; MG was not confirmed in the control trout by LC-MSn. MG was found in all of ten control basa samples at a level that was below the detection limit for the LC-VIS method. By LC-MSn analysis, the average background level of MG found in the basa samples was 0.30 ng/g. This quantity was subtracted from all basa recovery data presented in Table 1. In an attempt to eliminate this source of background contamination, nearly thirty basa samples obtained from retail and other sources were tested during the course of this study; however, a sample was not found that did not have a background level of malachite green.
As in the full salmon validation,1 the LC-VIS method detection limit was designated as 1 ng/g in accordance with the lowest calibration and spike levels. The average signal to noise ratio for 38 1 ng/g spikes in fish was 3.8. Lower concentration spikes were not included in the study. The day to day reproducibility of the method was measured by extracting five fish samples fortified with 2 ng/g of LMG on each of three days. Interday data for catfish, trout, tilapia, and shrimp is shown in Table 2.
The emphasis of the validation study was to determine LMG residues since this metabolite is expected to be the major compound found in tissue.3 Several MG spikes were also included in the study to determine how the extraction and DDQ oxidation might affect residual MG that may also be present in the tissue. Catfish, trout, tilapia, and shrimp were spiked with 100 µL of the 0.1 µg/mL MG2 working standard solution to produce samples fortified with 2 ng/g MG. For each species, one control and five spikes were extracted and analyzed. Average recoveries of MG were 64.4 ± 5.7 % for catfish, 78.1 ± 10.8 % for trout, 77.9 ± 3.4 % for tilapia, and 66.9 ± 5.2 % for shrimp. Previous studies also found satisfactory yet lower recoveries for MG as compared to LMG.1,2,11
Residue Confirmation by ND-APCI LC-MSn
This LC-MS method was developed to provide confirmation of the analysis of LMG residues. In order to achieve the most efficient use of laboratory resources, the same extracts prepared for the LC-VIS method were used for LC-MS analysis. In most cases, sample extracts were refrigerated and analyzed within five days of the extraction. Several samples were analyzed 15 to 60 days after the extraction. MG residues were confirmed in all cases.
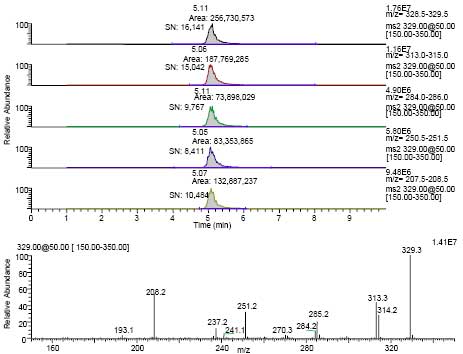
Malachite green is a charged (not protonated) species in solution with a molecular ion at m/z 329. The product ions include m/z 314 (M+ - CH3), m/z 313 (M+ - H-CH3), m/z 285 (M+ - NC2H6), m/z 251(M+ - C6H6), m/z 237 (M+ - C6H6 - CH3), and m/z 208 (M+ - C6H5 NC2H6). High collision energy was needed to obtain significant abundance of these ions. The amount of MG that could be detected and yield an adequate product ion spectrum was less than 1 pg. For a residue to be positively confirmed several criteria must be met.12 The retention time must match (within 5%) that of a standard. The product ion spectra must be visually similar to a standard with a minimum of unexplained background ions. In addition, the relevant product ions for MG should be present at an appreciable level and at the correct relative abundance as compared to a standard compound run that day. While this information could also be obtained from the MS2 scan, extracted ion chromatograms were generated for m/z 329, m/z 313-315, m/z 284-286, m/z 251, and m/z 208 in order to consistently determine these relative abundance ratios. The peaks at the correct retention time for MG in these extracted ion chromatograms were integrated, and the areas obtained were compared to that for the largest ion in the product spectrum (m/z 329). Examples of the of MS2 total ion chromatograms obtained from the analyses of tilapia samples (extracts from control, 2 ng/g fortified, and incurred fish) are shown in Figure 2. The extracted ion chromatograms and MS2 spectrum from a sample of retail basa contaminated with MG are illustrated in Figure 3.
Table 3 consists of a summary of all the retention time and relative abundance data that were obtained. For any one day's analysis, the variance of the retention times and relative abundances is much less than what is required by the confirmation criteria. The data obtained from the extracted samples matched that of solvent-based standards very well. Table 3 does indicate that the chromatographic retention and relative abundances of the product ions can vary somewhat over time. At one point during the study, the pressure in the ion trap increased which changed the relative abundances of the product ions. Lowering the collision energy slightly to a value of 48 for these analyses kept the ratios generally consistent with the rest of the validation data. For brevity, the qualitative results are presented as averages and standard deviations for each type of sample analyzed in a day. It is important to note, however, that each individual sample was evaluated to determine if confirmation criteria were met.
It is evident from these data that MG was confirmed in all samples fortified with LMG at the 1-10 ng/g level and in all extracts from fish that had been dosed with MG. While MG was not confirmed in any of the salmon control tissue extracts analyzed previously2, the residue was found at very low levels (< 0.25 ng/g) in a small percentage of the control extracts generated for this validation study. MG was confirmed in 2 of the 6 control catfish extracts, 1 of 9 tilapia samples, and 1 of 8 shrimp extracts. MG was not found in any of the trout or salmon control tissues in this study. While MG was also seen in one of the catfish samples by LC-VIS, the amounts present in the controls were, in general, too low to be detected by that method. The residue was not present in any of the solvent blanks (injected between standards and fish extracts in every LC-MSn sequence), indicating that instrument carry-over was not an issue. Also, MG was not found in any method reagent blanks tested. As with the LC-VIS analyses, MG was detected by LC-MSn in all extracts of basa purchased at the local market. MG was confirmed in 9 of 10 of these basa extracts, and detected, but not confirmed, in the tenth sample.
Residue Quantification by ND-APCI LC-MSn
The LC-VIS method was intended to be the primary method to routinely screen and quantify LMG and MG residues in numerous laboratory fish samples, without straining the resources of high-demand LC-MS instruments. However, a comparative analysis of quantification data by both the LC-VIS and the LC-MSn methods was also included in this study. The data for the recovery of LMG from tissues using LC-MSn with no-discharge APCI are shown in Table 1. The percent recovery was determined by measuring the amount of MG in the sample (peak area from the total ion chromatograms, not the extracted ion chromatograms) and comparing this amount to a calibration curve generated using standards in solvent. The standard curves were linear (r2 > 0.995) in this range of 1-20 ng/g. For samples with low amounts of MG (1 ng/g or lower), more accurate quantification could be obtained by using a slightly limited calibration range, so the 20 ng/g standard was excluded. In the present study, the recoveries by LC-MSn at all fortification levels ranged from 75 to 116 %, with relative standard deviations of 21 % or less. With a few exceptions, the two detection methods give comparable results with average recoveries equivalent within the margin of error (RSD) for each method. The precision for the LC-VIS determination was better than for the LC-MSn method, particularly at the 1 ng/g fortification level. However, these results indicate that LC-MSn is an acceptable alternative quantification method offering advantages for check analyses and in cases where an estimation of MG residues at levels lower than 1 ppb (LC-VIS detection limit) is required. For example, earlier experiments2 indicate that salmon tissue samples fortified with LMG at 0.25 ng/g could be detected and confirmed by LC-MSn with a recovery of 70 % and an RSD of 12 % , even though the levels were too low to be measured by LC-VIS. In addition, LC-MSn was used in this study to estimate the low amount of MG residues in the retail basa which was used as "control" tissue. It was found that this fish contained an average of 0.30 ng/g of LMG/MG residue (n = 9 samples extracted and analyzed over 5 days, RSD = 30 %). This quantity was subtracted from the calculated amount of residue found in fortified samples for both the LC-VIS and LC-MSn methods.
Incurred Tissues
Catfish, trout, tilapia, and salmon exposed to 10 µg/L MG in water for 1 hour were extracted after depuration times of 16, 16.5, 16.25, and 24 hours, respectively. The average (n = 5) sum of MG and LMG residues found in the tissues by LC-VIS was 32.2 ng/g in catfish, 27.1 ng/g in trout, 1.9 ng/g in tilapia, and 26.4 ng/g in salmon (Table 4). Since the concentrations found in catfish, trout, and salmon exceeded the calibration range of 1 to 20 ng/g, 2 mL of each final extract was diluted to 4 mL with mobile phase A and reanalyzed. These incurred samples were also confirmed and quantitatively analyzed by LC-MSn. As shown in Table 4, calculated concentrations by the two methods were within 9.5 % of each other. Five replicates were also analyzed of a commercially obtained sample of basa that tested positive for MG. An average concentration of 64 ng/g of LMG/MG residues was found in this "incurred" basa sample by both LC-VIS and LC-MSn (Table 4).
Conclusion
Two methods have been presented to determine the sum of LMG and MG residues in fish and shrimp with method detection limits of at least 1.0 ng/g (ppb). These methods rely on the in-situ conversion of LMG to MG using the oxidizing agent 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. LMG residues in catfish, trout, tilapia, basa, salmon, and shrimp were validated over the concentration range of 1.0 to 10.0 ppb, with overall recoveries (as MG) of 86 to 94 % with RSDs of 11 % or less. The method has also been used to determine MG in eel and broadhead clarias fish. ND-APCI LC-MSn was used for both confirmation and as an alternate quantitative method with a method detection limit of approximately 0.25 ng/g.
ACKNOWLEDGEMENTS
We gratefully thank Renate Reimschuessel and Charles Gieseker from the U.S. Food and Drug Administration Center for Veterinary Medicine for providing the incurred fish tissues used in this study, and to Steven Plakas of the U.S. Food and Drug Administration Gulf Coast Seafood Laboratory for providing the catfish.
REFERENCES
- W. C. Andersen, J. E. Roybal, S. B. Turnipseed, "Liquid Chromatographic Determination of Malachite Green and Leucomalachite Green (LMG) Residues in Salmon with in situ LMG Oxidation" (2005) J. AOAC Int. 88, 1292-1298.
- S. B. Turnipseed, W. C. Andersen, J. E. Roybal, "Determination and Confirmation of Malachite Green and Leucomalachite Green Residues in Salmon Using Liquid Chromatography/Mass Spectrometry with No-Discharge Atmospheric Pressure Chemical Ionization" (2005) J. AOAC Int. 88, 1312-1317.
- S. M. Plakas, D. R. Doerge, S. B. Turnipseed, "Disposition and Metabolism of Malachite Green and Other Therapeutic Dyes in Fish" In M. Beconi-Barker, W. H. Gingerich, and Smith D.J. (eds.), Xenobiotics in Fish. Plenum Press, New York City(1999) p. 149-166.
- S. J. Culp, F. A. Beland, "Malachite green: a toxicological review" (1996) J. Am. Coll. Toxicol. 15, 219-238.
- S. J. Culp, L. R. Blankenship, D. F. Kusewitt, D. R. Doerge, L. T. Mulligan, F. A. Beland, "Toxicity and Metabolism of Malachite Green and Leucomalachite Green during Short-Term Feeding to Fischer 344 rats and B6C3F1 Mice" (1999) Chem. Biol. Interact. 122, 153-170.
- R. A. Mittelstaedt, N. Mei, P. J. Webb, J. G. Shaddock, V. N. Dobrovolsky, L. J. McGarrity, S. M. Morris, T. Chen, F. A. Beland, K. J. Greenlees, R. H. Heflich, "Genotoxicity of Malachite Green and Leucomalachite Green in Female Big Blue B6C3F1 Mice" (2004) Mutat. Res. 561, 127-138.
- Annual Report on Surveillance for Veterinary Residues in Food in the UKfor 2001, 2002, and 2003, available at Veterinary Residues Committee.
- "Commission Decision 2004/25/EC as regards the setting of minimum required performance limits (MRPLs) for certain residues in food of animal origin" (2004) Off. J. Euro. Union, L6, 38-39.
- W. C. Andersen, J. E. Roybal, and S. B. Turnipseed, "Determination of Malachite Green and Leucomalachite Green in Salmon with In Situ Oxidation and Liquid Chromatography with Visible Detection" (2004) Laboratory Information Bulletin #4334, U.S. Food and Drug Administration, p. 1-13.
- S. B. Turnipseed, W. C. Andersen, J. E. Roybal Determination and Confirmation of Leucomalachite Green in Salmon using No-Discharge Atmospheric Pressure Chemical Ionization LC-MSn" (2004) Laboratory Information Bulletin #4333, U.S. Food and Drug Administration, p. 1-13.
- Roybal, J. E., Pfenning, A. P., Munns, R. K., Holland, D. C., Hurlbut, J. A., and Long, A. R., "Determination of malachite green and its metabolite, leucomalachite green, in catfish (Ictalurus punctatus) tissue by liquid chromatography with visible detection" (1995) J. AOAC Int. 78, 453-457.
- U.S. Food and Drug Administration. Guideline for Industry: Mass Spectrometry for Confirmation of the Identity of Animal Drug Residues. Fed. Reg. 68 (92) 25617. 2003.
| LMG Concentration (ng/g) |
LC-VIS Average MG Recovery (RSD, %) | Number of Samples by LC-VIS | LC-MSn Confirmed/ Analyzed |
LC-MSn Recovery (RSD, %) | |
|---|---|---|---|---|---|
| Catfish | 1 | 82.4 (6.8) | 10 | 5/5 | 92.2 (12.8) |
| 2 | 89.1 (7.2) | 22 | 7/7 | 97.6 (9.9) | |
| 4 | 82.2 (5.1) | 5 | 5/5 | 102.4 (4.0) | |
| 10 | 84.2 (11.1) | 10 | 5/5 | 98.0 (17.3) | |
| Trout | 1 | 87.7 (6.2) | 5 | 5/5 | 88.5 (4.2) |
| 2 | 89.1 (5.0) | 17 | 7/7 | 83.4 (13.3) | |
| 4 | 86.7 (5.8) | 5 | 5/5 | 81.6 (11.1) | |
| 10 | 84.9 (2.0) | 5 | 5/5 | 86.0 (5.8) | |
| Tilapia | 1 | 102.6 (5.2) | 10 | 10/10 | 82.3 (15.4) |
| 2 | 94.1 (6.8) | 17 | 12/12 | 94.0 (14.6) | |
| 4 | 84.3 (3.4) | 5 | 3/3 | 75.0 (11.8) | |
| 10 | 85.4 (0.6) | 5 | 3/3 | 78.7 (21.4) | |
| Basa | 1 | 91.5 (6.6) | 5 | 5/5 | 88.6 (12.4) |
| 2 | 76.1 (2.5) | 5 | 5/5 | 88.2 (6.2) | |
| 4 | 98.5 (5.8) | 5 | 5/5 | 109.4 (6.3) | |
| 10 | 95.9 (6.3) | 6 | 6/6 | 111.9 (6.6) | |
| Salmon | 2 | 95.1 (10.9) | 7 | 2/2 | 115.9 (10.7) |
| 4 | 91.2 (9.8) | 6 | 2/2 | 83.1 (5.4) | |
| 10 | 88.6 (8.5) | 2 | 2/2 | 75.5 (11.2) | |
| Shrimp | 1 | 95.5 (8.6) | 8 | 10/10 | 80.4 (20.8) |
| 2 | 91.4 (5.2) | 15 | 10/10 | 95.3 (8.2) | |
| 4 | 87.1 (1.2) | 5 | 3/3 | 96.1 (6.8) | |
| 10 | 79.4 (8.0) | 5 | 3/3 | 90.7 (5.4) |
| Recovery of 2 ng/g spikes (%) | Average (%) | RSD (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| SP1 | SP2 | SP3 | SP4 | SP5 | ||||
| Catfish | Day 1 | 83.3 | 94.3 | 94.3 | 99.7 | 95.6 | 95.6 | 6.5 |
| Day 2 | 86.0 | 87.2 | 84.9 | 83.0 | 86.5 | 85.5 | 1.9 | |
| Day 3 | 92.3 | 86.2 | 86.8 | 80.3 | 90.7 | 87.3 | 5.3 | |
| Trout | Day 1 | 85.5 | 87.0 | 78.1 | 88.9 | 91.5 | 86.2 | 5.9 |
| Day 2 | 90.1 | 85.5 | 90.9 | 88.5 | 89.1 | 88.8 | 2.3 | |
| Day 3 | 86.8 | 90.2 | 87.7 | 88.4 | 90.7 | 88.8 | 1.9 | |
| Tilapia | Day 1 | 91.3 | 89.9 | 83.4 | 95.0 | 83.6 | 88.6 | 5.7 |
| Day 2 | 107.0 | 99.1 | 98.5 | 99.5 | 94.6 | 99.7 | 4.5 | |
| Day 3 | 100.2 | 96.2 | 95.8 | 95.2 | 95.8 | 96.6 | 2.1 | |
| Shrimp | Day 1 | 95.3 | 88.4 | 85.1 | 86.9 | 88.5 | 88.8 | 4.4 |
| Day 2 | 91.7 | 103.9 | 97.4 | 93.8 | 88.5 | 95.1 | 6.2 | |
| Day 3 | 87.9 | 88.5 | 92.0 | 91.5 | 91.2 | 90.2 | 2.1 | |
| Type sample | N | Ret. Time | % Rel Abund. m/z 329 |
% Rel Abund. m/z 313-315 |
% Rel Abund. m/z 284-286 |
% Rel Abund. m/z 251 |
% Rel Abund. m/z 208 |
|---|---|---|---|---|---|---|---|
| Standards Day 1* | 8 | 5.21 ± 0.05 | 100 | 52 ± 5 | 20 ± 1 | 22 ± 2 | 37 ± 3 |
| 1 ng/g fortified | 5 | 5.24 ± 0.02 | 100 | 56 ± 6 | 21 ± 2 | 23 ± 2 | 38 ± 4 |
| 2 ng/g fortified | 5 | 5.25 ± 0.02 | 100 | 55 ± 5 | 21 ± 2 | 22 ± 1 | 37 ± 3 |
| 10 ng/g fortified | 5 | 5.23 ± 0.03 | 100 | 56 ± 6 | 21 ± 2 | 23 ± 2 | 39 ± 4 |
| Standards Day 2 | 7 | 5.13 ± 0.03 | 100 | 79 ± 8 | 33 ± 3 | 35 ± 4 | 56 ± 5 |
| 4 ng/g fortified | 5 | 5.12 ± 0.03 | 100 | 73 ± 5 | 29 ± 2 | 32 ± 3 | 51 ± 5 |
| Standards Day 3 | 7 | 5.30 ± 0.06 | 100 | 52 ± 6 | 23 ± 3 | 24 ± 2 | 38 ± 5 |
| Incurred | 5 | 5.25 ± 0.03 | 100 | 52 ± 4 | 21 ± 1 | 23 ± 2 | 36 ± 2 |
| 2 ng/g fortified | 2 | 5.25 ± 0.01 | 100 | 54 ± 6 | 21 ± 1 | 23 ± 1 | 37 ± 2 |
* on these days CE = 48
| Type sample | N | Ret. Time | % Rel Abund. m/z 329 |
% Rel Abund. m/z 313-315 |
% Rel Abund. m/z 284-286 |
% Rel Abund. m/z 251 |
% Rel Abund. m/z 208 |
|---|---|---|---|---|---|---|---|
| Standards Day 1 | 9 | 4.98 ± 0.06 | 100 | 70 ± 9 | 25 ± 3 | 29 ± 3 | 48 ± 4 |
| 1 ng/g fortified | 5 | 4.98 ± 0.01 | 100 | 68 ± 8 | 22 ± 3 | 25 ± 4 | 43 ± 6 |
| 2 ng/g fortified | 5 | 5.03 ± 0.02 | 100 | 71 ± 4 | 25 ± 3 | 29 ± 3 | 50 ± 6 |
| 4 ng/g fortified | 5 | 4.99 ± 0.02 | 100 | 73 ± 5 | 26 ± 2 | 30 ± 3 | 48 ± 4 |
| 10 ng/g fortified | 5 | 4.95 ± 0.02 | 100 | 67 ± 4 | 24 ± 2 | 28 ± 2 | 45 ± 4 |
| Standards Day 2 | 7 | 5.28 ± 0.03 | 100 | 68 ± 7 | 29 ± 2 | 30 ± 3 | 48 ± 7 |
| Incurred | 5 | 5.29 ± 0.01 | 100 | 70 ± 4 | 30 ± 2 | 31 ± 2 | 49 ± 4 |
| 2 ng/g fortified | 2 | 5.27 ± 0.02 | 100 | 66 ± 1 | 28 ± 1 | 28 ± 1 | 45 ± 2 |
| Type sample | N | Ret. Time | % Rel Abund. m/z 329 |
% Rel Abund. m/z 313-315 |
% Rel Abund. m/z 284-286 |
% Rel Abund. m/z 251 |
% Rel Abund. m/z 208 |
|---|---|---|---|---|---|---|---|
| Standards Day 1 | 8 | 5.30 ± 0.03 | 100 | 53 ± 3 | 22 ± 2 | 21 ± 8 | 38 ± 4 |
| 2 ng/g fortified | 10 | 5.31 ± 0.03 | 100 | 50 ± 4 | 21 ± 2 | 23 ± 2 | 36 ± 3 |
| Standards Day 2 | 8 | 5.33 ± 0.03 | 100 | 55 ± 7 | 22 ± 3 | 24 ± 3 | 38 ± 4 |
| 1 ng/g fortified | 5 | 5.35 ± 0.02 | 100 | 57 ± 3 | 24 ± 2 | 25 ± 2 | 40 ± 2 |
| 4 ng/g fortified | 3 | 5.33 ± 0.01 | 100 | 55 ± 7 | 23 ± 2 | 24 ± 4 | 38 ± 5 |
| 10 ng/g fortified | 3 | 5.31 ± 0.02 | 100 | 61 ± 6 | 24 ± 1 | 27 ± 4 | 43 ± 4 |
| Standards Day 3 | 7 | 5.30 ± 0.02 | 100 | 45 ± 5 | 18 ± 1 | 21 ± 3 | 32 ± 3 |
| Incurred | 5 | 5.30 ± 0.02 | 100 | 46 ± 4 | 20 ± 2 | 21 ± 2 | 31 ± 6 |
| 2 ng/g fortified | 2 | 5.29 ± 0.05 | 100 | 44 ± 2 | 19 ± 2 | 28 ± 1 | 31 ± 1 |
| Standards Day 4* | 8 | 5.19 ± 0.05 | 100 | 58 ± 4 | 22 ± 2 | 23 ± 2 | 40 ± 3 |
| 1 ng/g fortified | 5 | 5.26 ± 0.02 | 100 | 59 ± 6 | 22 ± 3 | 24 ± 3 | 41 ± 4 |
* on these days CE = 48
| Type sample | N | Ret. Time | % Rel Abund. m/z 329 |
% Rel Abund. m/z 313-315 |
% Rel Abund. m/z 284-286 |
% Rel Abund. m/z 251 |
% Rel Abund. m/z 208 |
|---|---|---|---|---|---|---|---|
| Standards Day 1* | 7 | 5.29 ± 0.11 | 100 | 59 ± 5 | 24 ± 2 | 26 ± 2 | 43 ± 3 |
| 2 ng/g fortified | 5 | 5.32 ± 0.01 | 100 | 52 ± 4 | 24 ± 2 | 23 ± 3 | 37 ± 3 |
| Standards Day 2 | 7 | 5.09 ± 0.04 | 100 | 61 ± 7 | 25 ± 3 | 26 ± 3 | 43 ± 3 |
| 4 ng/g fortified | 5 | 5.10 ± 0.04 | 100 | 66 ± 5 | 27 ± 3 | 28 ± 3 | 48 ± 5 |
| "Control" | 3 | 5.13 ± 0.01 | 100 | 57 ± 6 | 22 ± 2 | 22 ± 1 | 38 ± 2 |
| Standards Day 3 | 7 | 5.02 ± 0.05 | 100 | 62 ± 5 | 25 ± 2 | 26 ± 3 | 44 ± 5 |
| "Control" | 2 | 5.05 ± 0.01 | 100 | 68 ± 0 | 29 ± 0 | 30 ± 1 | 48 ± 6 |
| "Incurred" | 4 | 5.07 ± 0.03 | 100 | 68 ± 4 | 27 ± 2 | 31 ± 2 | 49 ± 1 |
| Standards Day 4 | 8 | 5.08 ± 0.02 | 100 | 49 ± 7 | 20 ± 2 | 21 ± 3 | 35 ± 4 |
| 1 ng/g fortified | 5 | 5.09 ± 0.04 | 100 | 53 ± 7 | 21 ± 3 | 22 ± 2 | 36 ± 4 |
| 10 ng/g fortified | 4 | 5.07 ± 0.01 | 100 | 51 ± 6 | 21 ± 2 | 23 ± 4 | 32 ± 6 |
* on these days CE = 48
| Type sample | N | Ret. Time | % Rel Abund. m/z 329 |
% Rel Abund. m/z 313-315 |
% Rel Abund. m/z 284-286 |
% Rel Abund. m/z 251 |
% Rel Abund. m/z 208 |
|---|---|---|---|---|---|---|---|
| Standards Day 1* | 8 | 5.22 ± 0.06 | 100 | 69 ± 9 | 26 ± 3 | 28 ± 4 | 47 ± 7 |
| 1 ng/g fortified | 2 | 5.24 ± 0.03 | 100 | 75 ± 5 | 25 ± 2 | 25 ± 2 | 43 ± 1 |
| 4 ng/g fortified | 2 | 5.22 ± 0.00 | 100 | 78 ± 0 | 29 ± 3 | 29 ± 0 | 51 ± 1 |
| 10 ng/g fortified | 2 | 5.24 ± 0.02 | 100 | 75 ± 4 | 28 ± 2 | 29 ± 2 | 48 ± 5 |
| Standards Day 2 | 7 | 5.33 ± 0.06 | 100 | 55 ± 3 | 22 ±1 | 24 ± 2 | 39 ± 2 |
| 2 ng/g fortified | 2 | 5.35 ± 0.06 | 100 | 58 ± 9 | 23 ± 2 | 24 ± 4 | 39 ± 6 |
| Incurred | 5 | 5.33 ± 0.01 | 100 | 60 ± 4 | 25 ± 2 | 27 ± 2 | 43 ± 2 |
| Type sample | N | Ret. Time | % Rel Abund. m/z 329 |
% Rel Abund. m/z 313-315 |
% Rel Abund. m/z 284-286 |
% Rel Abund. m/z 251 |
% Rel Abund. m/z 208 |
|---|---|---|---|---|---|---|---|
| Standards Day 1 | 8 | 5.34 ± 0.04 | 100 | 70 ± 5 | 29 ± 4 | 31 ± 3 | 49 ± 5 |
| 1 ng/g fortified | 5 | 5.33 ± 0.03 | 100 | 68 ± 3 | 28 ± 4 | 29 ± 5 | 49 ± 2 |
| 4 ng/g fortified | 3 | 5.32 ± 0.01 | 100 | 68 ± 11 | 28 ± 5 | 31 ± 4 | 46 ± 6 |
| 10 ng/g fortified | 3 | 5.34 ± 0.03 | 100 | 69 ± 6 | 29 ± 3 | 29 ± 4 | 50 ± 5 |
| Standards Day 2 | 8 | 5.29 ± 0.03 | 100 | 62 ± 6 | 25 ± 2 | 28 ± 3 | 42 ± 4 |
| 2 ng/g fortified | 10 | 5.26 ± 0.03 | 100 | 62 ± 6 | 26 ± 3 | 28 ± 3 | 46 ± 4 |
| Standards Day 3* | 8 | 5.19 ± 0.05 | 100 | 58 ± 4 | 22 ± 2 | 23 ± 2 | 40 ± 3 |
| 1 ng/g fortified | 5 | 5.26 ± 0.02 | 100 | 59 ± 6 | 22 ± 3 | 24 ± 3 | 41 ± 4 |
* on these days CE = 48
| Incurred Fish | Depuration Time | LMG/MG Found by LC-VIS | LMG/MG Found by LC-MSn |
|---|---|---|---|
| Catfish | 16 hours | 32.2 ng/g (6.8 % RSD) | 31.3 ng/g (8.7 % RSD) |
| Trout | 16.5 hours | 27.1 ng/g (5.2 % RSD) | 28.6 ng/g (3.8 % RSD) |
| Tilapia | 16.25 hours | 1.9 ng/g (7.2 % RSD) | 2.1 ng/g (14.2 % RSD) |
| Salmon | 24 hours | 26.4 ng/g (3.2 % RSD) | 27.4 ng/g (7.3 % RSD) |
| Basa | * | 64.3 ng/g (5.8 % RSD) | 64.7 ng/g (11.3 % RSD) |
* Positive retail sample found to contain malachite green.
Figure 1. Comparison of LC-VIS chromatograms; (a) 2 ppb MG standard,
(b) 2 ppb LMG spike (recovered as MG) in catfish, (c) control catfish,
(d) control trout, (e) control tilapia, (f) control basa, (g) control shrimp.
Figure 2. LC-MSn total ion chromatograms from MS2 of m/z 329.
Comparison of tilapia control (top), a 2 ng/g spike of the same tilapia tissue (middle),
and tilapia that had been dosed with MG (bottom). MG elutes at approximately 5.3 min.
Figure 3. Extracted ion chromatograms and product ion spectrum from the MG (m/z 329) product ion trace
in an extract from retail basa (diluted 1:5). Extracted ion ranges (from top to bottom):
m/z 329, m/z 313-315, m/z 284-286, m/z 251, m/z 208.