Laboratory Information Bulletin( LIB) 4333: Leucomalachite Green in Salmon
Determination and Confirmation of Leucomalachite Green in Salmon using No-Discharge Atmospheric Pressure Chemical Ionization LC-MSn
Volume 20, No. 11, November 2004
Sherri B. Turnipseed, Wendy C. Andersen, José E. Roybal
U.S. Food and Drug Administration, Animal Drugs Research Center, Denver, CO
This Laboratory Information Bulletin (LIB) is a tool for the rapid dissemination of laboratory methods which appear to work. It does not necessarily report completed scientific work. Users must assure themselves by appropriate validation procedures that LIB methods and techniques are reliable and accurate for their intended use. Reference to any commercial materials, equipment, or processes does not in any way constitute approval, endorsement, or recommendation by the Food and Drug Administration.
This document is also available in PDF (190 Kb).
ABSTRACT
An LC-MS method utilizing no-discharge atmospheric pressure chemical ionization (APCI), in conjunction with an ion trap instrument to generate product ion spectra, was developed to quantitate and confirm residues of leucomalachite green (LMG) in salmon tissue after conversion to the chromic malachite green (MG) in the extraction process. For the most efficient use of laboratory resources, the same extracts prepared for an LC-VIS method can be used for LC-MS analysis. The sample preparation procedure involves extraction of tissue into acetonitrile/buffer, partition of LMG residue into methylene chloride, conversion of LMG to MG using an organic oxidizing agent, followed by the isolation of MG on alumina/propyl sulfonic solid phase extraction cartridges. The validation of this method was performed by fortifying salmon with different levels of LMG, and then detecting the residue as MG. LC-MS conditions, including a comparison of electrospray and no-discharge atmospheric pressure chemical ionization (APCI), were evaluated and optimized. Confirmation criteria for retention time and product ion relative abundances that need to be met for positive identification of MG are provided. MG was not confirmed in any of the control tissue extracts, and all fortified samples analyzed in the validation of this method met the confirmation criteria as described. In addition to providing confirmatory data, this method can provide an alternative quantitation method for MG in salmon. The recoveries of LMG, measured as MG by this LC-MS method, at all fortification levels (1-10 ppb) were very high (87-109 %), with low relative standard deviations (<10%). The results agreed very closely with those obtained from the same extracts using the LC-VIS analysis procedure, indicating that matrix suppression was not an issue with this method.
The Laboratory Information Bulletin is a communication from the Division of Field Science, Office of Regulatory Affairs, U.S. Food and Drug Administration for the rapid dissemination of laboratory methods (or scientific regulatory information) which appear to solve a problem or improve an existing problem. In many cases, however, the report may not represent completed analytical work. The reader must assure, by appropriate validation procedures, that the reported methods or techniques are reliable and accurate for use as a regulatory method. Reference to any commercial materials, equipment, or process does not, in any way, constitute approval, endorsement, or recommendation by the U.S. Food and Drug Administration.
INTRODUCTION
Malachite green (MG) is a triphenylmethane dye that is sometimes administered illegally as a fungicide in aquaculture facilities. Malachite green undergoes a conversion to the reduced leuco form in fish tissue. Leucomalachite green (LMG) is lipophilic and can remain in fatty tissue for extended periods of time.1 Studies have shown that MG is potentially carcinogenic.2 Therefore persistent residues in food fish is a concern, and analytical methods are needed to monitor for low levels of these drugs in fish tissue. MG has a strong chromophore (618 nm) and several methods have been developed that make use of LC with visible absorbance to detect residues as the chromic form. Because the residue is found primarily as the reduced leuco compound in tissue, oxidation is required to convert this residue to the colored form of the drug. Conversion to MG can be achieved with an LC post-column reaction, usually on a lead oxide reactor bed,3-5 which will measure MG and LMG residues separately. Alternatively, an oxidation step can be incorporated into the extraction procedure to measure all residues as the chromic form. Recently, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) has been used to successfully oxidize LMG residues in salmon to MG for subsequent LC-VIS analysis.6,7
In addition to developing determinative methods to quantitate the amount of residues present, it is also important to qualitatively identify the drugs. Because of its excellent sensitivity and selectively, mass spectrometry (MS) is universally recognized as the best technique for confirmation of identity.8,9 There are several methods available for the MS analysis of these drugs: GC-MS,10 and LC-MS using a particle beam,11 electrospray (ESI),5,6,12-14 and no-discharge atmospheric pressure chemical ionization (APCI)15,16. Confirmatory fragment or product ions can be obtained by electron ionization,10,11 in-source CID of an LC-MS signal,5,15 or by performing LC-MS-MS with a triple quadrupole6,12,13 or ion trap detector.14,16
In the method reported here, we have developed an LC-MS procedure which takes advantage of the sensitivity of no-discharge APCI and the specificity of an ion trap detector to develop and validate a complete LC-MS method for confirmation and determination of LMG residues in salmon tissue. The method is complementary to the LC-VIS method developed in our laboratory,7 with the same efficient extraction method and pre-column oxidation of the LMG residue to the charged, chromic MG.
EXPERIMENTAL
Standard and Sample Preparation
Standards and samples were prepared exactly as for the LC-VIS procedure. An abbreviated description of the preparation of reagents, standards and of the extraction are provided here, but a more detailed description of the procedure can be found in that reference.7
Reagents and Laboratory Equipment
- Solvents - Acetonitrile (ACN) HPLC and UV spectro-grade (Burdick & Jackson Laboratories, Inc, Muskegon, MI), methanol (EMD Chemicals, Gibbstown, NJ), methylene chloride (Aldrich, St Louis, MO), or equivalent.
- Water de-ionized - purified to 18.2 MΩ cm (mega-ohm-cm) with a Milli-Q Plus water purification system (Millipore, Bedford, MA).
- 0.1% Formic Acid – mobile phase for LC-MSn. Pipette 1 mL formic acid (Baker Analyzed, Phillipsburg NJ) into a 1000 mL graduated cylinder. Bring to volume with deionized water.
- Acetate buffer - prepare by adjusting 1L of a 0.1M ammonium acetate solution (7.7 g ammonium acetate (Sigma, St. Louis, MO) in 1000mL water) to pH 4.5 with acetic acid (JT Baker, Phillipsburg, NJ) (ca 8 mL) and 5 mL of 1M p-toluene sulfonic acid (p-TSA, Sigma), this is equivalent to 5mM p-TSA.
- 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) - stock solution of DDQ (Sigma) was prepared at a concentration of 0.01M in acetonitrile. A 1:10 dilution in ACN was made for the DDQ working solution (0.001M DDQ).
- Solid Phase Extraction (SPE) cartridges – alumina columns (ALN-SPE), Bakerbond alumina SPE, neutral, 6 mL, 500 mg (JT Baker); propylsulfonic acid (PRS-SPE), Bond Elut LRC propylsulfonic acid SPE, 500 mg (Varian, Palo Alto, CA).
- Other reagents - diethylene glycol (Fisher Chemicals, Fairlawn, NJ), alumina (80-200 mesh, EM Science), and hydroxylamine hydrochloride (0.25 g/mL in DI water, Sigma), p-TSA (1 M solution in water).
- Other laboratory equipment. - blender/homogenizer, vortex mixer, centrifuge (compatible with 50 mL tubes, refrigerated at 0 ºC), rotary evaporator, SPE manifold, volumetric glassware and pipettes, adjustable pipettors, 150 mL pear shaped boiling flasks, 250 mL separatory funnels, 50 mL centrifuge tubes, 2 mL LC vials.
Standard Preparation
- Malachite Green (MG). A stock solution at a concentration of 100 µg/mL in methanol was prepared by weighing out malachite green oxalate standard (Sigma). The amount was corrected for purity, and also for MG-oxalate, which contains only 7.109 mg of MG for 10 mg of MG-oxalate.
- Calibration curve standards. Prepared by making initial dilutions of this MG stock solution in methanol to make intermediate standards at 1.0 µg/mL and 0.1µg/mL. These standards are then diluted to 5 mL with 50:50 acetonitrile: acetate buffer to make final solutions in the range of 0, 1, 2, 4, 10 and 20 ppb of MG.
- Leucomalachite green (LMG). LMG (Aldrich) standards were used to fortify the samples. A stock solution (100 µg/mL) was prepared in methanol. Fortification standards at 1µg /mL and 0.1 µg/mL were prepared by making serial dilutions of the stock standard in methanol. Appropriate aliquots of these fortification standards (50-200 µL) were added to 5 g of tissue to generate samples fortified at 1-10 ppb.
Preparation and Extraction of Salmon Samples
Fish tissue was coarsely chopped and blended with dry ice in blender/homogenizer until the material had consistency of fine powder. The carbon dioxide was allowed to dissipate overnight, and the tissue was then sealed in Whirl-Pak bags and stored (-20 ºC) until analysis. Five g of sample were weighed into 50 mL centrifuge tubes, and the tissue was homogenized by vortex mixing (30 s) with 5 mL acetate buffer, 100µL of 1 M p-TSA solution, and 1 mL of hydroxylamine (0.25g/mL). The tissue was then extracted using acetonitrile (25 mL) with vigorous shaking for 30 s. Then 5-6 g alumina was added and sample was vigorously shaken again for 15 s. The sample was centrifuged (0 ºC, 4000 rpm) and the supernatant was added to a 250 mL separatory funnel containing 50 mL of water and 2 mL of diethylene glycol. A second extraction with another 25 mL of ACN was performed, and the resulting supernatant was added to the first. A liquid-liquid extraction to partition the residue into methylene chloride was done twice with 25 mL aliquots of methylene chloride. The methylene chloride was collected in a 150 mL pear-shaped flask, and then the solvent was removed by roto-evaporation. The residue was dissolved in 3 mL of ACN and then oxidized to MG with 3 mL of a 0.001M DDQ solution for 30 min. The chromic form of the residue then underwent further clean-up using alumina and propylsulfonic acid solid phase extraction cartridges (SPEs conditioned first with methanol and then with ACN). The alumina column was then placed on top of the PRS cartridge. The extract was applied to the alumina column and allowed to elute onto the PRS SPE at a rate of approximately 4 mL/min. The PRS-SPE column was washed with acetonitrile, and MG was eluted from the PRS-SPE with 4 mL of 50:50 acetonitrile:ammonium acetate buffer and brought up to a volume of 5 mL with the same solution.
LC-MS Instrumental Conditions
The LC-MS system consisted of an Agilent (Avondale, PA) 1100 LC interfaced to a ThermoElectron (San Jose, CA) Finnigan DECA-XP Plus Ion Trap MS with either an electrospray or an atmospheric pressure chemical ionization (APCI) source. XCaliber (Version 1.3) was the software used to operate both instruments. The LC-MS was tuned by flowing MG standard solution (0.5 ng/µL of each MG in 50/50 water/methanol) at a rate of 10 µL/min using a syringe pump into a stream of 700 µL/min 63/37 0.1% formic/ACN via a T fitting. Using this procedure, electrospray and no-discharge APCI ionization were investigated. Source parameters such as lens voltages, gas flows, vaporizer and capillary temperatures, and collision energy were also optimized in this manner. Typical MS parameters for no-discharge APCI were determined to be: corona discharge 0 µA; vaporizer temperature, 400° C; capillary temperature, 220° C; capillary voltage, 40V; sheath gas, 70; auxiliary gas, 40. For ESI the following were used: source voltage, 5kV ; sheath gas, 80; auxiliary gas, 20; capillary voltage, 40V; capillary temperature, 350° C. The number of prescans was equal to two and the maximum inject time was set to 500 ms for MS2 scans in both no-discharge APCI and ESI tune files. The MS acquisition programs consisted of a MS2 scan of m/z 329 with isolation width of 2 amu, collision energy = 50, Q = 0.25, activation time = 30 ms, range = m/z 150-350.
The final instrument procedure consisted of a 15 minute LC program which was isocratic 63/37 0.1%formic/ACN for the first 10 minutes, followed by a quick ramp to 100% ACN from 10 to 10.5 minutes, a column wash at 100% ACN from 10.5-12 minutes, ramping back to 63/37 0.1% formic/ACN from 12 to 12.5 minutes, and equilibrating at that composition for the final 2.5 min. The LC column was a YMC phenyl 3-4-5 cartridge (3 µ, 120 Å, 4.0 x 50 mm, P/N PH12S030504WTA, Waters Corp., Milford, MA), with a guard cartridge insert (4.0 x 20mm) of the same phase. The column was maintained at 30° C. The mobile phase flow was 700 µL/min. Ten µL injections were used with a needle wash of water. The divert valve was switched to the MS at 1 min and to waste at 9.8 min.
The treatment of the data varies depending on whether qualitative (confirmatory) or quantitative data were being evaluated. For qualitative assessment, individual ion transition chromatograms were generated and the resulting chromatographic peaks were integrated. Relative abundances were calculated from these peak areas and compared to contemporary standards. For quantitative assessment the area counts of the MG peak from the total ion chromatogram, not the extracted ion chromatograms, were used. Peak areas could be calculated using either the qualitative (Qual Browser®) or quantitative (Quan Browser®) software programs, but should be consistent for both standards and samples.
RESULTS AND DISCUSSION
This LC-MS method was developed to provide confirmatory data for the analysis of LMG residues in salmon, as well as an alternative detection mode for the determination of this residue. In order to achieve the most efficient use of laboratory resources, the same extracts prepared for the LC-VIS7 method were used for LC-MS analysis. This extraction procedure includes an oxidation step to convert LMG residues to MG, which is more easily detected by both LC-VIS and LC-MS. The validation of this method was performed by fortifying salmon with different levels of LMG, and then detecting the residue as MG.
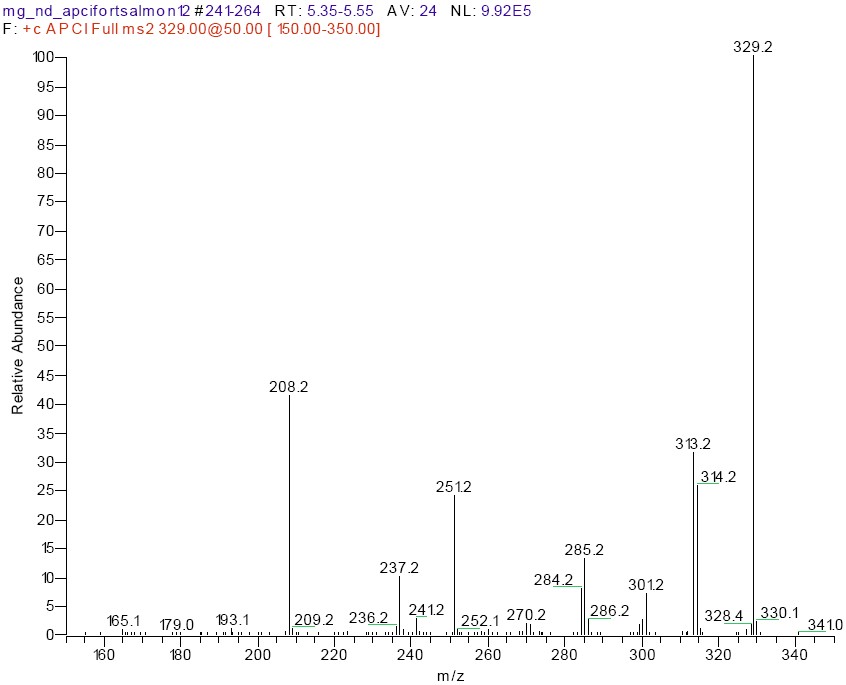
In this study, electrospray ionization was compared to no-discharge atmospheric pressure chemical ionization for MS2 analysis of MG residues on an ion trap LC-MS instrument. Malachite green is a charged (not protonated) species in solution with a molecular ion at m/z 329. The product ion spectra obtained by no-discharge APCI (Figure 1) was virtually identical to that obtained by ESI, although the response for the same amount of compound injected was considerably (40x) higher in the case of no-discharge APCI. The product ions generated were similar to what has been observed before with other MS techniques including electron ionization,10,11 in-source collision activated dissociation,5,15 or in other true MS-MS experiments such as ion trap14,16 or triple quadrupole.6,12,13 The product ions include m/z 314 (M+ - CH3), m/z 313 (M+ - H-CH3), m/z 285 (M+ - NC2H6), m/z 251(M+ - C6H6), m/z 237 (M+ - C6H6 - CH3), and m/z 208 (M+ - C6H5 NC2H6). High collision energy was needed to obtain significant abundance of these ions, regardless of whether the molecular ions were generated with ESI or no-discharge APCI. The relative intensity of the product ions could be increased by using a longer ion trap activation time, but it was determined that the sensitivity and spectral quality were sufficient with the standard value of 30 ms. The amount of MG that could be detected and yield an adequate product ion spectrum was less than 1 pg with no-discharge APCI and 5 pg with ESI. A lower flow rate (200 µL) with a 2 x 100 mm LC column of a similar (phenyl) stationary phase increased the ESI to some extent, but the response for MG using no-discharge APCI was still substantially higher. No-discharge APCI LC-MS has been previously described for the analysis of animal drug residues17,18 including MG,15,16 as well as other compounds of biological interest.19,20
The extracts from the farm-raised salmon used for the fortification study contained significant matrix peaks. These peaks were detected by both LC-VIS and the LC-MS2 m/z 329 product ion trace obtained with either no-discharge APCI or ESI. When the control extracts were analyzed by LC-MS with a full scan acquisition program, the signal from the matrix components was very intense with ions at relatively high mass (i.e. m/z 535, 461). There was not a significant contribution from the ion at m/z 329 in the MS spectra of these chromatographic peaks, but the background from these interferences still contributed to the m/z 329 product ion trace. Adjustments were made in the chromatographic program to separate MG from these co-extracted matrix compounds. A comparison of the chromatograms generated from the m/z 329 product ion for a MG standard, control salmon tissue, and tissue spiked at 2 ppb can be seen in Figure 2. MG was added to a finished control salmon tissue extract at the 10 ppb level (end spike) and this was compared to a 10 ppb MG standard in solvent. The calculated area for MG in the end spike was 95% of that in the standard, indicating that matrix suppression or enhancement was not an issue with this chromatographic system. These interference peaks were observed primarily with fresh farm-raised salmon, and were not seen in fresh wild salmon or in canned (wild-caught) salmon.
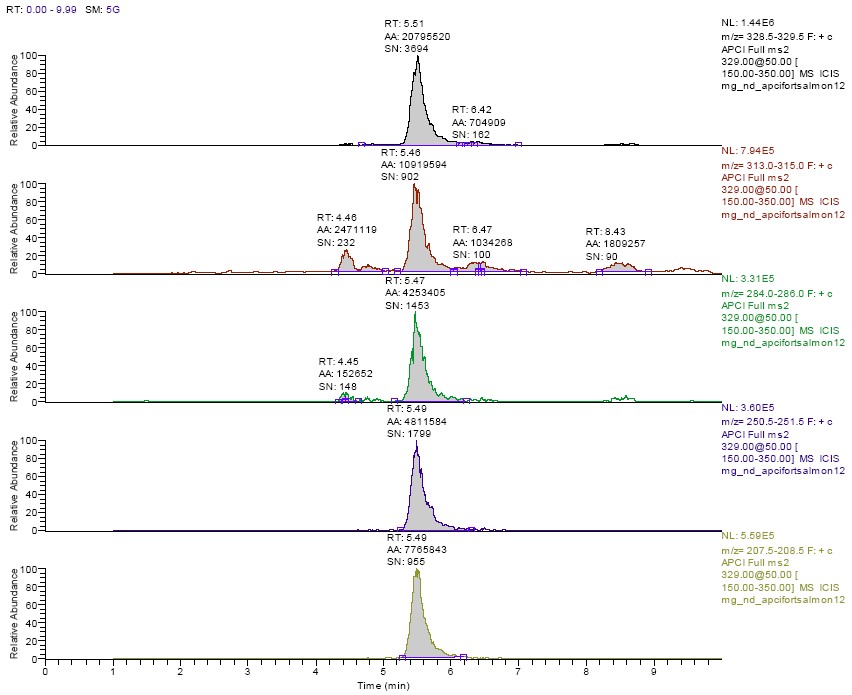
For a residue to be positively confirmed several criteria must be met.8 The retention time must match (within 5%) that of a standard. The product ion spectra must be visually similar to a standard with a minimum of unexplained background ions. In addition, the relevant product ions for MG should be present at an appreciable level and at the correct relative abundance as compared to standard compound run that day. While this information could also be obtained from the MS2 scan (Figure 1), extracted ion chromatograms were generated for m/z 329, m/z 313-315, m/z 284-286, m/z 251, and m/z 208 in order to consistently determine these relative abundance ratios. The peaks at the correct retention time for MG in these extracted ion chromatograms were integrated, and the areas obtained were compared to that for the biggest ion in the product spectrum (m/z 329). An example of the extracted ion chromatograms obtained from the analysis of a 2 ppb spike is shown in Figure 3. In addition, the retention time and relative abundance data from one day's analysis of a set of 2 ppb spikes is shown in Table 1. These data show that the relative abundance of these products ions is consistent, and that the spectral data obtained from the extracted salmon samples matches that of solvent-based standards very well. It is also evident from these data that MG was not confirmed in any of the control tissue extracts, and that all fortified samples met the confirmation as described.
The data for the recovery of LMG from salmon tissue using LC-MSn with no-discharge APCI are also shown in Table 2. The percent recovery was determined by measuring the amount of MG in the sample (peak area from the total ion chromatograms, not the extracted ion chromatograms) and comparing this amount to a calibration curve generated using standards in solvent. The standard curves were linear in this range 1-20 ppb (10- 200 pg injected on-column) with correlation coefficients averaging 0.9983 (average of 6 curves containing 7 standards each). The recoveries at all fortification levels were very high, with low relative standard deviations. The results matched very closely with those obtained from the same extracts using the LC-VIS analysis procedure,7 further indicating that matrix suppression was not an issue with this method. In addition, one set of samples was fortified with MG, instead of LMG, at levels ranging from 1 -10 ppb. The recovery on these samples was somewhat lower (66%, with 17% RSD), but still acceptable.
A limited number of samples (six control samples, twelve samples with LMG at the 1 (n=1), 2 (n=7), 4 (n=2), and 10 (n=2) ppb level) were also analyzed, qualitatively and quantitatively, using electrospray ionization. The same chromatographic program was used for this analysis, with a tune file optimized for MG using the electrospray ionization source. While the response for MG was considerably less using this technique, it was still possible to detect and confirm the residue in fortified salmon extracts down to the 1 ppb level. The calculated recoveries for fortified samples, as compared to a standard curve analyzed using the same ionization method, were also acceptable and comparable to the no-discharge APCI data. However, the quality of the MS2 spectra and the signal-to-noise for the extracted ion chromatograms were lower than those obtained using no-discharge APCI.
In conclusion, this method demonstrates that LC-MS with no-discharge APCI ionization, in conjunction with an ion trap instrument to generate product ion spectra, can successfully quantitate and confirm residues of LMG in salmon tissue after conversion to MG in the extraction process.
| Sample | Retention Time (min) |
% Rel Abund. m/z 329 |
% Rel Abund. m/z 313-315 |
% Rel Abund. m/z 284-286 |
% Rel Abund. m/z 251 |
% Rel Abund. m/z 208 |
|---|---|---|---|---|---|---|
| Standard 20 ppb | 5.46 | 100 | 63 | 25 | 28 | 45 |
| Standard 10 ppb | 5.47 | 100 | 56 | 23 | 25 | 41 |
| Standard 4 ppb | 5.55 | 100 | 64 | 25 | 28 | 46 |
| Standard 4 ppba | 5.54 | 100 | 64 | 25 | 29 | 48 |
| Standard 2 ppb | 5.56 | 100 | 60 | 24 | 29 | 47 |
| Standard 1 ppb | 5.51 | 100 | 61 | 21 | 26 | 42 |
| Average of Standards | 5.54 | 100 | 61 | 24 | 27 | 44 |
| Buffer | NDb | |||||
| Control Salmon | ND | |||||
| Fortified Salmon 2 ppb-1 | 5.52 | 100 | 62 | 22 | 25 | 42 |
| Fortified Salmon 2 ppb-2 | 5.53 | 100 | 54 | 19 | 23 | 39 |
| Fortified Salmon 2 ppb-3 | 5.54 | 100 | 60 | 20 | 25 | 42 |
| Fortified Salmon 2 ppb-4 | 5.53 | 100 | 64 | 24 | 27 | 42 |
| Fortified Salmon 2 ppb-5 | 5.58 | 100 | 63 | 23 | 26 | 43 |
| Standard 20 ppb | 5.58 | 100 | 62 | 24 | 27 | 44 |
| Standard 4 ppb | 5.61 | 100 | 56 | 21 | 26 | 42 |
a second initial calibration validation standard
b not detected
| Samples | Average Recovery | Relative Standard Deviation | # Confirmed of # Analyzed |
|---|---|---|---|
| Controla | Not applicable | Not applicable | 0 of 11 |
| 1 ppb LMG | 109.2 | 9.2 | 5 of 5 |
| 2 ppb LMGb | 93.4 | 9.2 | 15 of 15 |
| 4 ppb LMG | 93.5 | 8.7 | 5 of 5 |
| 10 ppb LMGc | 87.8 | 9.2 | 15 of 15 |
a controls were analyzed over several days, at least one control per set of spikes
b 2 ppb spikes were run over 3 days (sets of 5 spikes/day)
c 10 ppb spikes were run over 3 days (sets of 5 spikes/day). Canned salmon, rather than fresh farm-raised, was used for one set (5 samples) of fortifications.
Figure 1. Product Ion Spectra of MG (m/z 329) from 2 ppb spike using No-Discharge APCI
Figure 2. Total ion chromatograms from MS2 of m/z 329. Comparison of 2 ppb standard (top), farm raised salmon control (middle), and 2 ppb spike of the same salmon tissue (bottom).
MG elutes at approximately 5.5 min.
Figure 3. Extracted ion chromatograms from the MG (m/z 329) product ion trace in a 2 ppb fortified salmon extract.
Extracted ion ranges (from top to bottom): m/z 329, m/z 313-315, m/z 284-286, m/z 251, m/z 208.
REFERENCES
- Plakas, S. M., Doerge, D. R., and Turnipseed, S. B. Disposition and Metabolism of Malachite Green and Other Therapeutic Dyes in Fish, p. 149-166. In M. Beconi-Barker, W. H. Gingerich, and Smith D.J. (eds.), Xenobiotics in Fish . Plenum Press, New York City.1999.
- Culp, S. J. NTP technical report on the toxicity studies of malachite green chloride and leucomalachite green (CAS Nos. 569-64-2 and 129-73-7) administered in feed to F344/N rats and B6C3F1 mice. Toxic. Rep. Ser.1-F10. 2004.
- Allen, J. L., and Meinertz, J. R. Post-column reaction for simultaneous analysis of chromatic and leuco forms of malachite green and crystal violet by high-performance liquid chromatography with photometric detection. J. Chromatogr. 536, 217-222. 1991.
- Roybal, J. E., Pfenning, A. P., Munns, R. K., Holland, D. C., Hurlbut, J. A., and Long, A. R. Determination of malachite green and its metabolite, leucomalachite green, in catfish (Ictalurus punctatus) tissue by liquid chromatography with visible detection. J. AOAC Int. 78, 453-457. 2004.
- Tarbin, J. A., .Barnes, K. A., Bygrave, J., and Farrington, W. H. H. Screening and confirmation of triphenylmethane dyes and their leuco metabolites in trout muscle using HPLC-VIS and ESP-LC-MS. Analyst 123, 2567-2571. 1998.
- Van Rhijn, J. A, Mulder, P. P. J., van Baardewijk, te Brinke E. M., and Lasaroms, J. J. P. Confirmatory Analysis of Traces of Malachite Green and Leucomalachite Green in Muscle Tissue of Atlantic Salmon. Presented at Euroresidue V, Noordwijkerhout, The Netherlands, 5-10-2004.
- Andersen, W. C., Roybal, J. E., and Turnipseed, S. B. Determination of Malachite Green and Leucomalachite Green in Salmon with In Situ Oxidation and Liquid Chromatography with Visible Detection. Laboratory Information Bulletin. 2004.
- U.S. Food and Drug Administration. Guideline for Industry: Mass Spectrometry for Confirmation of the Identity of Animal Drug Residues. Fed. Reg. 68 (92) 25617. 2003.
- European Union. Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. 2002/657/EC. 2002.
- Turnipseed, S. B., Roybal, J. E., Hurlbut, J. A., and Long, A. R. Confirmation of Malachite Green Residues in Catfish Tissue by GC/MS. J. AOAC Int. 78, 971-977. 1995.
- Turnipseed, S. B., Roybal, J. E., Rupp, H. S., Hurlbut, J. A., and Long, A. R. Particle Beam LC/MS of Triphenylmethane Dyes: Application to Confirmation of Malachite Green in Incurred Catfish Tissue. J. Chromatogr. B Biomed. Sci. Appl. 670, 55-62. 1995.
- Halme, K, Lindfors, E., and Petonen, K. Determination of Malachite Green Residues in Rainbow Trout muscle with Liquid Chromatography and Liquid Chromatography Coupled with Tandem Mass Spectrometry. Food Addit. Contamin. 21, 641-648. 2004.
- Scherpenisse, P., and Bergwerff, A. A. Determination of residues of malachite green in finfish by liquid chromatography tandem mass spectrometry . Anal. Chim. Acta. 485, in press. 2004.
- Turnipseed, S. B., Roybal, J. E., Pfenning, A. P., and Kijak, P. J. Use of liquid chromatography-ion trap mass spectrometry to screen and confirm drug residues in aquacultured products. Anal. Chim. Acta 483, 373-386.2003.
- Doerge, D. R., Churchwell, M. I., Gehring, T., Pu, Y.M., and Plakas, S. M. Analysis of malachite green and metabolites in fish using liquid chromatography atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 12, 1625-1634.1998.
- Li, H., Cui, W., Turnipseed, S. B., and Kijak, P. J. LC-MSn Screening and Confirmation of Multi-Class Antibiotic Residues in Shrimp. 2004. Proceedings of the 52th ASMS Conference on Mass Spectrometry. Nashville, TN, 5-20-2004.
- Doerge, D. R., Churchwell, M. I, Rushing, L. G., and Bajic, S. Confirmation of gentian violet and its metabolite leucogentian violet in catfish muscle using liquid chromatography combined with atmospheric pressure ionization mass spectrometry. Rapid Commun. Mass Spectrom. 10,1479-1484.1996.
- Turnipseed, S. B., Roybal, J. E., Andersen, W. C., and Kuck, L. R. Analysis of Avermectin Residues in Milk by LC-MS Using an Atmospheric Pressure Chemical Ionization/Atmospheric Pressure Photoionization Source. Anal. Chim. Acta 485, in press. 2004.
- Cristoni, S, Bernardi, L. R., Biuno, I., and Guidugli, F. Analysis of peptides using partial (no discharge) atmospheric pressure chemical ionization conditions with ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 16, 1686-1691. 2002.
- Sakairi, M., and Kato, Y. Multi-atmospheric pressure ionization interface for LC-MS. J. Chromatogr. A 794, 391-406. 1998.