Drug Trials Snapshots: KOSELUGO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the KOSELUGO Prescribing Information for complete information.
KOSELUGO (selumetinib)
(ko-SEL-yuh-go)
AstraZeneka
Approval date: April 10, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
KOSELUGO is used to to treat children 2 years of age and older with a type of tumor called plexiform neurofibroma or PN that occurs in a rare disease called neurofibromatosis type 1 (NF1). KOSELUGO is used in patients whose tumors cause symptoms and could not be surgically removed.
How is this drug used?
KOSELUGO is a capsule taken two times a day.
What are the benefits of this drug?
In the trial, 33 of 50 patients (66%) who received KOSELUGO experienced partial tumor shrinkage. Of these patients, 82% had a tumor shrinkage lasting 12 months or longer.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the trial based on ORR.
Table 1. Efficacy Results From the Triala
|
Efficacy Parameter |
KOSELUGO |
|
Overall Response Rateb |
|
|
Overall Response Rate, n (%) |
33 (66%) |
|
95% CI |
(51, 79) |
|
Complete Response |
0 |
|
Confirmed Partial Response, n (%)c |
33 (66%) |
|
Duration of Response |
|
|
DoR ≥ 12 months, n (%) |
27 (82%) |
CI – confidence interval, DoR – duration of response.
a The ORR assessment was conducted by a single NCI reviewer who was a SPRINT investigator and who evaluated all PN imaging from patients enrolled at all trial sites.
b Responses required confirmation at least 3 months after the criteria for first response were met.
c Complete response: disappearance of the target lesion; Partial response: decrease in target PN volume by ≥ 20% compared to baseline.
KOSELUGO Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: KOSELUGO worked similarly in males and females.
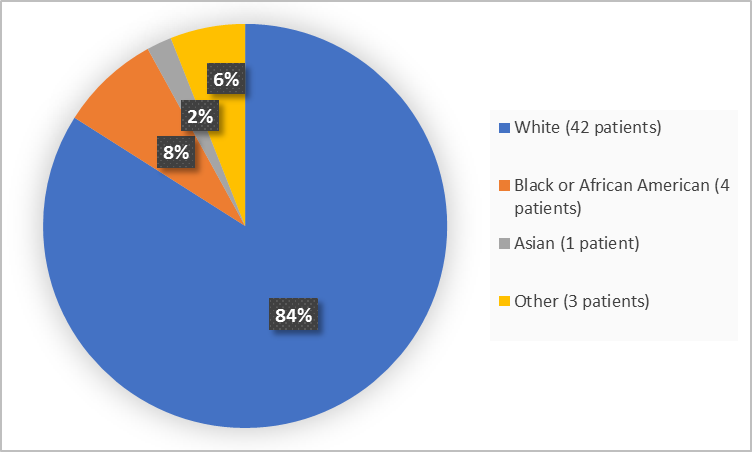
- Race: The majority of patients were White. Therefore, differences among races could not be determined.
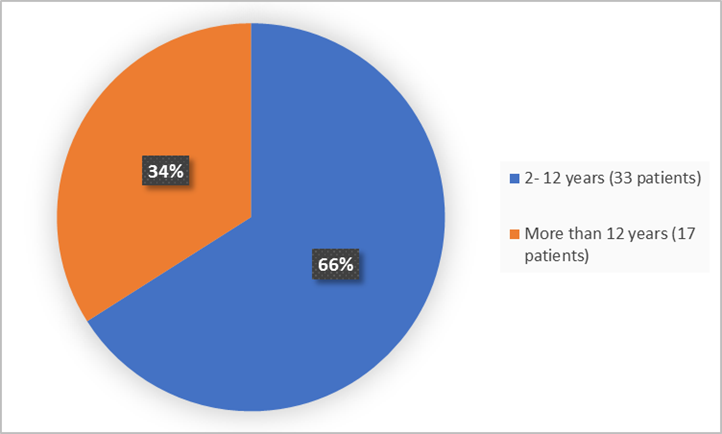
- Age: KOSELUGO worked similarly in children younger and older than12 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The investigator assessed demographic subgroup ORR results are presented in Table 2.
Table 2. Analysis of Confirmed Overall Response Rate by Demographic Subgroups
|
Demographic Subgroup |
KOSELUGO (N=50) |
|
Sex |
|
|
Male (n=30) |
60 (41, 77) |
|
Female (n=20) |
75 (51, 91) |
|
Race |
|
|
White (n=42) |
69 (53, 82) |
|
All Other (n=8) |
50 (16, 84) |
|
Age |
|
|
≤ 12 years (n=33) |
70 (51, 84) |
|
> 12 years (n=17) |
59 (33, 82) |
FDA Review
What are the possible side effects?
KOSELUGO may cause serious side effects including:
- weaking of the heart,
- eye problems that can result in loss of sight,
- gastrointestinal problems (diarrhea, bleeding),
- severe skin rashes,
- increased blood levels of enzyme creatinine phosphokinase, and
- increased risk of bleeding when taken with vitam E.
The most common side effects of KOSELUGO are vomiting, rash, abdominal pain, diarrhea, nausea, dry skin, fatigue, muscule and bone pain, fever, acne, mouth sores, headache, nail infection, and itching.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in the clinical trial.
Table 3. Adverse Reactions (≥ 20%) in Patients Who Received KOSELUGO in the Trial
|
Adverse Reaction |
KOSELUGO |
|
|
All Grades |
Grade ≥ 3 |
|
|
Gastrointestinal |
||
|
Vomiting |
82 |
6 |
|
Abdominal pain1 |
76 |
0 |
|
Diarrhea |
70 |
16 |
|
Nausea |
66 |
2 |
|
Stomatitis2 |
50 |
0 |
|
Constipation |
34 |
0 |
|
Skin and Subcutaneous Tissue |
||
|
Rash (all) 3 |
80 |
6 |
|
Dry skin |
60 |
0 |
|
Rash acneiform4 |
50 |
4 |
|
Paronychia5 |
48 |
6 |
|
Pruritis |
46 |
0 |
|
Dermatitis6 |
36 |
4 |
|
Hair changes7 |
32 |
0 |
|
Musculoskeletal and Connective Tissue |
||
|
Musculoskeletal pain8 |
58 |
0 |
|
General |
||
|
Fatigue9 |
56 |
0 |
|
Pyrexia |
56 |
8 |
|
Edema10 |
20 |
0 |
|
Nervous System |
||
|
Headache |
48 |
2 |
|
Respiratory, Thoracic and Mediastinal |
||
|
Epistaxis |
28 |
0 |
|
Renal and Urinary System |
||
|
Hematuria |
22 |
2 |
|
Proteinuria |
22 |
0 |
|
Metabolism and Nutrition |
||
|
Decreased appetite |
22 |
0 |
|
Cardiac System |
||
|
Decreased ejection fraction |
22 |
0 |
|
Sinus tachycardia |
20 |
0 |
|
Infections |
|
|
|
Skin infection11 |
20 |
2 |
* All events were Grade 3.
1 Abdominal pain includes abdominal pain; abdominal pain upper
2 Stomatitis includes stomatitis; mouth ulceration
3 Rash (all) includes dermatitis acneiform; rash maculo-papular; erythema; rash pustular; rash; urticaria; exfoliative rash; rash pruritic; rash erythematous
4 Rash (acneiform) includes dermatitis acneiform
5 Paronychia includes paronychia, nail infection
6 Dermatitis includes dermatitis; dermatitis atopic; dermatitis diaper; eczema; seborrheic dermatitis; skin irritation
7 Hair changes include alopecia, hair color change
8 Musculoskeletal pain includes pain in extremity; back pain; neck pain; musculoskeletal pain
9 Fatigue includes fatigue, malaise
10 Edema includes peripheral swelling, edema, localized edema
11 Skin infection includes skin infection; abscess; cellulitis; impetigo; staphylococcal skin infection
KOSELUGO Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of overall side effects was similar in males and females.
- Race: The majority of patients were White. Therefore, differences in side effects among races could not be determined.
- Age: The occurrence of overall side effects was similar in children younger and older that 12 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The following table summarizes adverse reactions by demographic subgroups.
Table 4. Subgroup Analysis of Adverse Reactions in Subgroups
|
Adverse Reactions |
KOSELUGO (N=50) |
|
|
All Grades |
Grade ≥ 3 |
|
|
Overall |
98 |
62 |
|
Sex |
||
|
Male (n=30) |
100 |
70 |
|
Female (n=20) |
95 |
50 |
|
Race |
||
|
White (n=42) |
100 |
62 |
|
AA/Asian (n=5) |
100 |
60 |
|
Age |
||
|
<12 years (n=32) |
97 |
66 |
|
> 12 years (n=18) |
100 |
56 |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved KOSELUGO based on a clinical trial (NCT01362803) of 50 children 2-18 years of age with NF1.
The trial was conducted at 4 sites in the United States.
Figure 1 summarizes how many boys and girls were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 summarizes patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Review
The figure below summarizes the age groups of patients in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA Review
Figure 4 summarizes patients by ethnicity in the trial.
Figure 4. Baseline Demographics by Ethnicity
FDA Review
The table below summarizes baseline demographics for the trial that supported the approval of KOSELUGO.
Table 5. Trial Demographics
|
Demographics |
KOSELUGO |
|
Sex, n (%) |
|
|
Male |
30 (60) |
|
Female |
20 (40) |
|
Race, n (%) |
|
|
White |
42 (84) |
|
Black or African American |
4 (8) |
|
Asian |
1 (2) |
|
Other |
3 (6) |
|
Age (years) |
|
|
Mean (SD) |
10.3 (3.92) |
|
Median (min, max) |
10 (3.5-17) |
|
Age Group,n (%) |
|
|
≤12 years |
33 (66) |
|
>12 years |
17 (34) |
|
Ethnicity, n (%) |
|
|
Not Hispanic or Latino |
45 (90) |
|
Hispanic or Latino |
2 (4) |
|
Unknown |
2 (4) |
|
Not reported |
1 (2) |
|
Region,n (%) |
|
|
USA |
50 (100) |
FDA Review
How were the trials designed?
The trial enrolled patients with NF1 whose PN(s) were causing significant symptoms and could not be surgically removed. The enrolled patients received KOSELUGO capsule twice daily until disease progression or unacceptable toxicity.
The benefit of KOSELUGO was assessed by the percentage of patients with complete or partial shrinkage of the tumors (overall response rate or ORR).
How were the trials designed?
The efficacy and safety of KOSELUGO were evaluated in an open-label, multicenter, single arm trial (SPRINT Phase II Stratum 1, NCT01362803). Eligible patients were required to have NF1 with inoperable PN, defined as a PN that could not be completely removed without risk for substantial morbidity due to encasement of, or close proximity to, vital structures, invasiveness, or high vascularity of the PN. Patients received KOSELUGO 25 mg/m2 orally twice daily until disease progression or unacceptable toxicity.
The major efficacy outcome measure was ORR, defined as the percentage of patients with complete response (defined as disappearance of the target PN) or confirmed partial response (defined as ≥ 20% reduction in PN volume confirmed at a subsequent tumor assessment within 3 6 months). An additional efficacy outcome measure was DoR.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.