Drug Trial Snapshot: XOSPATA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to XOSPATA Prescribing Information for complete information.
XOSPATA (gilteritinib)
zoh spah' tah
Astellas Pharma
Approval date:November 28, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
XOSPATA is used to treat adults with acute myeloid leukemia (AML) that have a mutation in a gene called FLT3 and whose disease has come back or has not improved after previous treatment(s).
AML is a rapidly progressing cancer that forms in the bone marrow and results in an increased number of white blood cells in the bloodstream.
How is this drug used?
XOSPATA is a tablet. Three tablets (total of 120 mg) are taken once a day.
What are the benefits of this drug?
Twenty-nine patients (21%) of the 138 patients who received XOSPATA experienced no evidence of disease and full or partial recovery of blood counts after treatment. Of the 106 patients who required red blood cell or platelet transfusions at the start of treatment with XOSPATA, 31 percent became transfusion-free for at least 56 days.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results established on the basis of the rate of complete remission (CR)/complete remission with partial hematologic recovery (CRh), the duration of CR/CRh (DOR), and the rate of conversion from transfusion dependence.
Table 2: Efficacy Results in Patients with Relapsed or Refractory Acute Myeloid Leukemia (AML)-Trial 1
|
Remission Rate |
XOSPATA |
|---|---|
|
CR*/CRh† n/N (%) |
29/138 (21) |
|
95% CI‡ |
14.5, 28.8 |
|
Median DOR§ (months) |
4.6 |
|
Range (months) |
0.1 to 15.8¶ |
|
CR* n/N (%) |
16/138 (11.6) |
|
95% CI‡ |
6.8, 18.1 |
|
Median DOR§ (months) |
8.6 |
|
Range (months) |
1 to 13.8 |
|
CRh† n/N (%) |
13/138 (9.4) |
|
95% CI‡ |
5.1, 15.6 |
|
Median DOR§ (months) |
2.9 |
|
Range (months) |
0.1 to 15.8¶ |
CI: confidence interval; NE: not estimable; NR: not reached; Only responses prior to HSCT were included in response rate.
*CR was defined as an absolute neutrophil count ≥1.0 x 109/L, platelets ≥100 x 109/L, normal marrow differential with <5% blasts, must have been red blood cells, platelet transfusion independent and no evidence of extramedullary leukemia.
†CRh was defined as marrow blasts <5%, partial hematologic recovery absolute neutrophil count ≥0.5 x 109/L and platelets ≥50 x 109/L, no evidence of extramedullary leukemia and could not have been classified as CR.
‡The 95% CI rate was calculated using the exact method based on binomial distribution.
§DOR was defined as the time from the date of either first CR or CRh until the date of a documented relapse of any type.
Deaths were counted as events.
¶Response was ongoing.
XOSPATA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: XOSPATA worked similarly in men and women.
- Race: XOSPATA worked similarly in White and Asian patients. Differences in how well the drug worked among other races could not be determined because of the small number of patients of other races.
- Age: XOSPATA worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by demographic subgroups. These analyses were exploratory and therefore should be interpreted with caution.
Table 3. Subgroup Analyses of Complete Remission Rate by Sex, Race, and Age
|
Subgroup |
CR/CRh (%) |
95% CI |
|---|---|---|
|
Sex |
||
|
Men (n=64) |
10 (15.6) |
(7.8, 26.9) |
|
Women (n=74) |
19 (25.7) |
(16.2, 37.2) |
|
Race |
||
|
White (n=82) |
22 (26.8) |
(17.6, 37.8) |
|
Black or African American (n=10) |
0 (0) |
(NA, NA) |
|
Asian (n=37) |
7 (18.9) |
(8.0, 35.2) |
|
Other (n=9) |
0 (0) |
(NA, NA) |
|
Age (years) |
||
|
<65 (n=85) |
15 (17.7) |
(10.2, 27.4) |
|
≥65 (n=53) |
14 (26.4) |
(15.3, 40.3) |
Adapted from FDA Review
What are the possible side effects?
XOSPATA may cause serious side effects including condition called posterior reversible encephalopathy syndrome (PRES). PRES is characterized by seizures, quickly worsening headache, and mental status and vision changes. Other serious side effects include heart rhythm problems (because of QT prolongation), inflammation of pancreas and a harm to unborn baby.
The most common side effects of XOSPATA are muscle and joint pain, fatigue and elevated liver enzymes.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions and laboratory abnormalities that occurred in patients from the combined three clinical trials.
Table 4. Adverse Reactions Reported in ≥10% (Any Grade) or ≥5% (Grade 3-5) of Patients with Relapsed or Refractory AML
|
Body System |
XOSPATA |
|
|---|---|---|
|
Any Grade |
Grade ≥3* |
|
|
Musculoskeletal and Connective Tissue Disorders |
||
|
Myalgia/arthralgia† |
123 (42) |
13 (5) |
|
Investigations |
||
|
Transaminase increased‡ |
121 (41) |
47 (16) |
|
Bilirubin increase § |
31 (11) |
14 (5) |
|
General Disorders and Administration Site Conditions |
||
|
Fatigue/malaise¶ |
116 (40) |
14 (5) |
|
Fever# |
103 (35) |
13 (5) |
|
Edemaþ |
100 (34) |
5 (2) |
|
Noninfectious diarrheaß |
99 (34) |
8 (3) |
|
Constipation |
80 (27) |
2 (<1) |
|
Nausea |
78 (27) |
4 (1) |
|
Stomatitisà |
77 (26) |
11 (4) |
|
Vomitingè |
58 (20) |
3 (1) |
|
Respiratory, Thoracic and Mediastinal Disorders |
||
|
Dyspneað |
98 (34) |
36 (12) |
|
Cough |
74 (25) |
1 (<1) |
|
Skin and subcutaneous tissue disorders |
||
|
Rashø |
87 (30) |
8 (3) |
|
Infections and Infestations |
||
|
Pneumoniaý |
89 (30) |
66 (23) |
|
Sepsis₤ |
43 (15) |
41 (14) |
|
Vascular disorders |
||
|
Hypotension¥ |
60 (21) |
21 (7) |
|
HypertensionΠ|
30 (10) |
17 (6) |
|
Nervous System Disorders |
||
|
Headacheɶ |
60 (21) |
4 (1) |
|
DizzinessÐ |
57 (20) |
1 (<1) |
|
Dysgeusia |
31 (11) |
0 |
|
Renal and urinary disorders |
||
|
Renal impairmentA |
54 (19) |
11 (4) |
|
Gastrointestinal Disorders |
||
|
Abdominal painB |
50 (17) |
5 (2) |
|
Metabolism and Nutrition disorders |
||
|
Decreased appetite |
44 (15) |
6 (2) |
|
Psychiatric Disorders |
||
|
Insomnia |
42 (14) |
1 (<1) |
*Grade 3-5 includes serious, life-threatening and fatal adverse reactions
†Grouped terms: arthralgia, back pain, bone pain, myalgia, musculoskeletal pain, neck pain, non-cardiac chest pain, pain and pain in extremity
‡Grouped terms: aspartate aminotransferase increased, alanine aminotransferase increased, transaminases increased, liver function test increased, hepatic failure, hepatocellular injury and hepatotoxicity
§Grouped terms: blood bilirubin increased and hyperbilirubinemia
¶Grouped terms: asthenia, fatigue and malaise
#Grouped terms: body temperature increased and fever
þGrouped terms: edema, face edema, fluid retention, generalized edema, localized edema, edema peripheral, peripheral swelling and swelling face
ßGrouped terms: diarrhea and diarrhea hemorrhagic
àGrouped terms: aphthous ulcer, mucosal inflammation, mouth hemorrhage, mouth ulceration, oral mucosal blistering, oral mucosal erythema, stomatitis and tongue ulceration
èGrouped terms: hematemesis and vomiting
ðGrouped terms: acute respiratory failure, acute respiratory distress syndrome, dyspnea, dyspnea exertional, hypoxia, pulmonary edema, respiratory distress, respiratory failure, tachypnea and wheezing
øGrouped terms: dermatitis, dermatitis bullous, dermatitis contact, drug eruption, dermatitis exfoliative, eczema asteatotic, lichen planus, erythema, palmar-plantar erythrodysethesia syndrome, photosensitivity reaction, psoriasis, rash, rash erythematous, rash follicular, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, seborrheic dermatitis, skin exfoliation and toxic skin eruption
ýGrouped terms: pneumonia, lung infection, pneumonia fungal, respiratory syncytial virus infection, respiratory tract infection, lung infiltration, organizing pneumonia, lower respiratory tract infection bacterial, pneumonia aspiration, pneumonitis, interstitial lung disease, lower respiratory tract infection and pneumonia viral
₤Grouped terms: sepsis, bacteremia, septic shock, bacterial sepsis and neutropenic sepsis
¥Grouped terms: blood pressure decreased, hypotension and orthostatic hypotension
ŒGrouped terms: blood pressure increased, hypertension and orthostatic hypertension
ɶGrouped terms: headache and tension headache
ÐGrouped terms: dizziness, dizziness postural and vertigo
AGrouped terms: acute kidney injury, blood creatinine increased, chronic kidney disease, oliguria, renal disorder, renal failure, renal impairment, renal injury and renal tubular necrosis
BGrouped terms: abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, epigastric discomfort and gastrointestinal pain
XOSPATA Prescribing Information
Table 5. Most Common (≥20%) New or Worsening Laboratory Abnormalities Reported in Patients with Relapsed or Refractory AML
|
Parameter |
XOSPATA |
||
|---|---|---|---|
|
Any Grade |
Grade ≥3* |
|
|
|
Creatinine increased |
273 (94) |
10 (3) |
|
|
Hyperglycemia |
252 (86) |
26 (9) |
|
|
Hypertriglyceridemia |
237 (81) |
18 (6) |
|
|
Alanine aminotransferase increased |
229 (78) |
35 (12) |
|
|
Aspartate aminotransferase increased |
228 (78) |
28 (10) |
|
|
Alkaline phosphatase increased |
189 (65) |
3 (1) |
|
|
Hypocalcemia |
179 (61) |
15 (5) |
|
|
Hypoalbuminemia |
169 (58) |
10 (3) |
|
|
Creatine kinase increased |
157 (54) |
14 (5) |
|
|
Hypophosphatemia |
141 (48) |
36 (12) |
|
|
Hypokalemia |
103 (35) |
25 (9) |
|
|
Hyponatremia |
93 (32) |
36 (12) |
|
|
*Grade 3-5 includes serious, life-threatening and fatal adverse reactions. |
|||
XOSPATA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was higher in women.
- Race: The occurrence of side effects was similar between White and Asian patients. Differences in the occurrence of side effects among other races could not be determined because of the small number of patients of other races.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Tables below summarize adverse events during the clinical trials by sex, age and race subgroups in safety population.
Table 6. Subgroup Analyses of Common Adverse Events by Sex
|
Adverse Event |
Men (N=138) |
Women (N=154) |
Risk |
||
|---|---|---|---|---|---|
|
|
n |
% |
n |
% |
|
|
Urinary tract infection |
0 |
0 |
21 |
14 |
-14 |
|
Stomatitis |
27 |
20 |
50 |
32 |
-13 |
|
Hypertransaminasemia |
49 |
36 |
72 |
47 |
-11 |
|
Blood alkaline phosphatase increased |
17 |
12 |
36 |
23 |
-11 |
|
Hypokalemia |
24 |
17 |
43 |
28 |
-11 |
|
Edema |
40 |
29 |
60 |
39 |
-10 |
|
Epistaxis |
31 |
22 |
17 |
11 |
11 |
Table 7. Subgroup Analyses of Common Adverse Events by Race
|
Adverse Event |
White (N=180) |
Asian (N=71) |
Black (N=16) |
Risk Difference* |
|||
|---|---|---|---|---|---|---|---|
|
|
n |
% |
n |
% |
n |
% |
|
|
Dizziness |
34 |
19 |
11 |
15 |
7 |
44 |
-25 |
|
Pneumonia |
59 |
33 |
17 |
24 |
9 |
56 |
-23 |
|
Hypomagnesaemia |
26 |
14 |
8 |
11 |
6 |
38 |
-23 |
|
Pericarditis |
6 |
3 |
3 |
4 |
4 |
25 |
-22 |
|
Encephalopathy |
9 |
5 |
3 |
4 |
4 |
25 |
-20 |
|
Cough |
43 |
24 |
20 |
28 |
7 |
44 |
-20 |
|
Hematoma |
10 |
6 |
1 |
1 |
4 |
25 |
-19 |
|
Nausea |
46 |
26 |
19 |
27 |
7 |
44 |
-18 |
|
Decreased appetite |
24 |
13 |
14 |
20 |
5 |
31 |
-18 |
|
Vomiting |
36 |
20 |
11 |
15 |
6 |
38 |
-18 |
|
Urinary retention |
4 |
2 |
0 |
0 |
3 |
19 |
-17 |
|
Arrhythmia |
27 |
15 |
7 |
10 |
5 |
31 |
-16 |
|
Constipation |
52 |
29 |
21 |
30 |
2 |
13 |
16 |
|
Anemia |
83 |
46 |
28 |
39 |
4 |
25 |
21 |
*Risk difference for Black and White subgroups
Table 8. Subgroup Analyses of Common Adverse Events by Age
|
Adverse Event |
Age <65 Years |
Age ≥65 Years |
Risk |
||
|---|---|---|---|---|---|
|
|
n |
% |
n |
% |
|
|
Sepsis |
18 |
10 |
25 |
21 |
-11 |
|
Hyponatremia |
14 |
8 |
21 |
18 |
-10 |
|
Fall |
12 |
7 |
18 |
15 |
-8 |
|
Epistaxis |
23 |
13 |
25 |
21 |
-8 |
|
Muscular weakness |
10 |
6 |
15 |
13 |
-7 |
|
Pneumonia |
48 |
28 |
41 |
34 |
-7 |
|
Leukocytosis |
4 |
2 |
10 |
8 |
-6 |
|
Hyperglycaemia |
19 |
11 |
20 |
17 |
-6 |
|
Diarrhea |
57 |
33 |
46 |
39 |
-6 |
|
Anemia |
67 |
39 |
52 |
44 |
-5 |
|
Pancreatitis |
2 |
1 |
7 |
6 |
-5 |
|
Oedema |
56 |
32 |
44 |
37 |
-5 |
|
Retinal haemorrhage |
14 |
8 |
4 |
3 |
5 |
|
Mouth haemorrhage |
10 |
6 |
1 |
1 |
5 |
|
Hypocalcaemia |
32 |
19 |
15 |
13 |
6 |
|
Dizziness |
38 |
22 |
19 |
16 |
6 |
|
Hypokalaemia |
44 |
25 |
23 |
19 |
6 |
|
Rash |
56 |
32 |
31 |
26 |
6 |
|
Hyperbilirubinaemia |
23 |
13 |
8 |
7 |
7 |
|
Leukopenia |
27 |
16 |
10 |
8 |
7 |
|
Paraesthesia |
20 |
12 |
5 |
4 |
7 |
|
Hypertransaminasaemia |
77 |
45 |
44 |
37 |
8 |
|
Pain |
19 |
11 |
4 |
3 |
8 |
|
Pruritus |
19 |
11 |
4 |
3 |
8 |
|
Electrocardiogram QT prolonged |
20 |
12 |
4 |
3 |
8 |
|
Nausea |
52 |
30 |
26 |
22 |
8 |
|
Headache |
42 |
24 |
18 |
15 |
9 |
|
Hypomagnesaemia |
32 |
19 |
11 |
9 |
9 |
|
Neutropenia |
101 |
58 |
58 |
49 |
10 |
|
Vomiting |
43 |
25 |
13 |
11 |
14 |
FDA Review
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved XOSPATA based on evidence from three clinical trials of 292 patients with AML whose disease has come back or has not improved after previous treatment(s).
In Trial 1 (NCT02421939), there were 138 patients and all of them had a certain type of mutation (FLT3-mutation) which was confirmed using an FDA-approved test. The population from this trial provided data that was used to assess the benefit of XOSPATA (called efficacy population). Demographics are presented in Table 10 under more info.
FDA also used data from other two trials where patients were treated with the same dose of XOSPATA as in the efficacy trial. That population (together with population from Trial 1) provided data for assessment of side effects (called safety population). Demographics are presented in the figures below and in Table 9 under MORE INFO.
All trials were conducted in United States, Asia and Europe.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Review
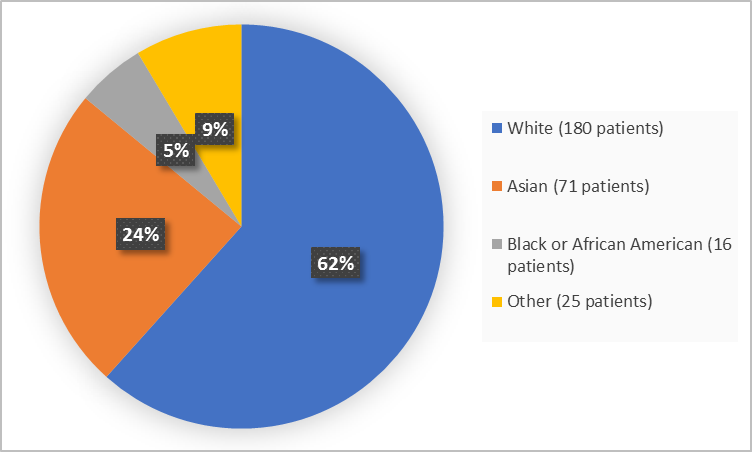
Figure 2 and Table 1 below summarize the percentage of patients by race in the clinical trial.
F
igure 2. Baseline Demographics by Race
FDA Review
Table 1. Baseline Demographics by Race
|
Race |
Number of Patients |
Percentage |
|---|---|---|
|
White |
180 |
62 |
|
Black or African American |
16 |
5 |
|
Asian |
71 |
24 |
|
Other or missing |
25 |
9 |
FDA Review
Figure 3 summarizes how many patients of certain age were enrolled in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The table below summarizes demographics of all patients in the three clinical trials combined who received XOSPATA at recommended dose (safety population).
Table 9. Baseline Demographics of Patients in the Clinical Trials (safety population)
|
Demographic Characteristic |
XOSPATA (N=292) |
|
|---|---|---|
|
|
n |
% |
|
Sex |
||
|
Men |
138 |
47% |
|
Women |
154 |
53% |
|
Race |
||
|
White |
180 |
62% |
|
Asian |
71 |
24% |
|
Black or African American |
16 |
5% |
|
Other or missing |
25 |
9% |
|
Age |
||
|
<65 years |
173 |
59% |
|
≥65 years |
119 |
41% |
|
≥75 years |
39 |
13% |
|
Ethnicity |
||
|
Hispanic or Latino |
13 |
5% |
|
Not Hispanic or Latino |
279 |
95% |
|
Region |
||
|
North America |
167 |
57% |
|
Asia |
67 |
23% |
|
Europe |
58 |
20% |
FDA Review
The table below presents demographics of patients who participated in trial used to establish XOPATA efficacy (efficacy population).
Table 10. Baseline Demographics of Patients in the Clinical Trial 1 (efficacy population)
|
Demographic Characteristic |
XOSPATA (N=138) |
|
|---|---|---|
|
|
n |
% |
|
Sex |
||
|
Men |
64 |
46% |
|
Women |
74 |
54% |
|
Race |
||
|
White |
82 |
60% |
|
Asian |
37 |
27% |
|
Black or African American |
10 |
7% |
|
Other or missing |
7 |
5% |
|
Age Category |
||
|
<65 years |
85 |
62% |
|
≥65 years |
53 |
38% |
|
≥75 years |
19 |
14% |
|
Ethnicity |
||
|
Hispanic or Latino |
6 |
4% |
|
Not Hispanic or Latino |
127 |
94% |
|
Region |
||
|
North America |
74 |
54% |
|
Asia |
36 |
26% |
|
Europe |
28 |
20% |
FDA Review
How were the trials designed?
There was one trial that evaluated the benefits of XOSPATA in patients with AML whose disease has come back or has not improved after previous treatment(s). All patients had a certain type of mutation (FLT3-mutation) which was confirmed using an FDA-approved test.
Patients received XOSPATA once a day until disease worsened or unacceptable toxicity.
The benefit of XOSPATA was evaluated by measuring:
- how many patients reached complete remission (no evidence of disease) with full or partial recovery of blood counts after treatment,
- how long those patients remained in remission,
- and the percentage of patients who no longer required transfusions after treatment.
Additional two trials in patients with AML whose disease has come back or has not improved after previous treatment(s) provided data for XOSPATA side effects. All patients in that population (safety population) received XOSPATA at the recommended dose, but not all of them had FLT-3 mutation.
How were the trials designed?
The safety and efficacy of XOSPATA were established in three open-label, randomized, clinical trials of adult patients with relapsed or refractory AML who received XOSPATA orally at dose of 120 mg daily until disease progression or unacceptable toxicity.
The efficacy was established in one of the three trials where all patients had confirmed FLT3 mutation. Efficacy endpoints were the rate of complete remission (CR)/complete remission with partial hematologic recovery (CRh), the duration of CR/CRh (DOR), and the rate of conversion from transfusion dependence to transfusion independence in the trial.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION