Patient Labeling Resources
For Industry
On this page
- Who is the audience for this webpage?
- What types of patient labeling are available in the United States?
- What are Medication Guides?
- What are Patient Package Inserts?
- What are Instructions for Use?
- What is Patient Medication Information?
- Contact information
Who is the audience for this webpage?
FDA’s patient labeling specific resources on this webpage are primarily directed to industry staff who develop patient labeling for prescription drugs. For assistance on how to navigate this webpage and the associated FDA labeling resource webpages for human prescription drugs see video. For more information about:
- Other prescription drug labeling resources for industry such as those for the Prescribing Information, carton and container labeling, generic drug labeling, biological product labeling, labeling databases, and product databases visit FDA’s Labeling Resources for Human Prescription Drugs.
- Drug labeling resources for healthcare professionals, patients, and caregivers, visit Frequently Asked Questions about Labeling for Prescription Medicines.
What types of patient labeling are available in the United States?
FDA-approved patient labeling includes:
Not all prescription drugs are required to have FDA-approved patient labeling.
In May 2023, FDA published a proposed rule about a new type of FDA-approved patient labeling, known as Patient Medication Information (PMI).
Consumer medication information (CMI) is written information for patients and caregivers about a prescription drug that is developed by an individual(s) or organization other than the drug company. FDA does not review or approve CMI, and the drug company does not review CMI.
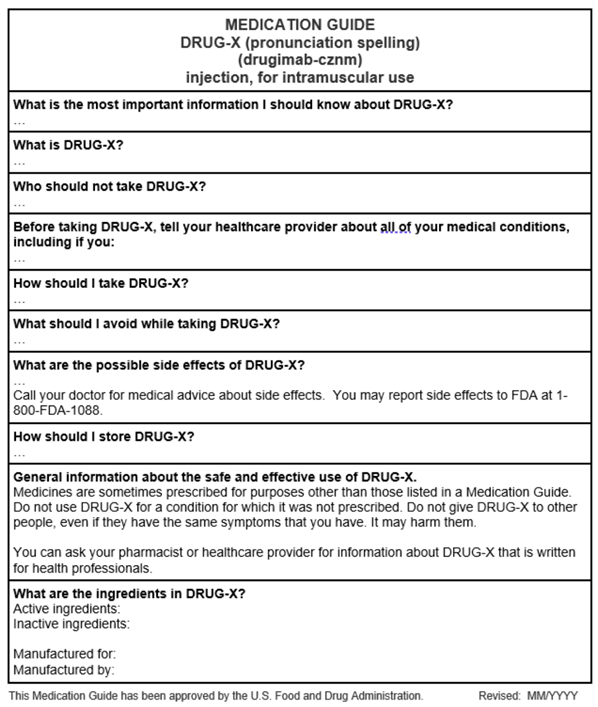
What are Medication Guides?
A Medication Guide is patient labeling that is part of the FDA-approved prescription drug labeling for certain prescription drugs when the FDA determines that:
- Patient labeling could help prevent serious adverse reactions
- The drug has serious risk(s) (relative to benefits) of which patients should be made aware because information concerning the risk(s) could affect patients' decision to use, or to continue to use, the product, or
- Patient adherence to directions for use is crucial to the drug’s effectiveness.
Medication Guides (MGs) are developed by applicants, approved by FDA, and required to be distributed to patients. The medication guide shall be dispensed to the patient (or to the patient’s agent) in paper form when the product is dispensed; however, the patient may also request electronic delivery of the MG in lieu of the printed form.
MG resources include:
- MG regulations available at 21 CFR 208
- Medication Guides - Distribution Requirements and Inclusion in Risk Evaluation and Mitigation Strategies (REMS) (final guidance)
- A guidance that has specific recommendations for the MG is the following: Child-Resistant Packaging Statements in Drug Product Labeling (final guidance).
Below see an example MG template that provides a general overview of the MG (this MG does not contain any product-specific information). Consider using the enclosed Sample Template for Medication Guides when developing a Medication Guide (this sample template may not contain all the Medication Guide requirements or guidance recommendations).
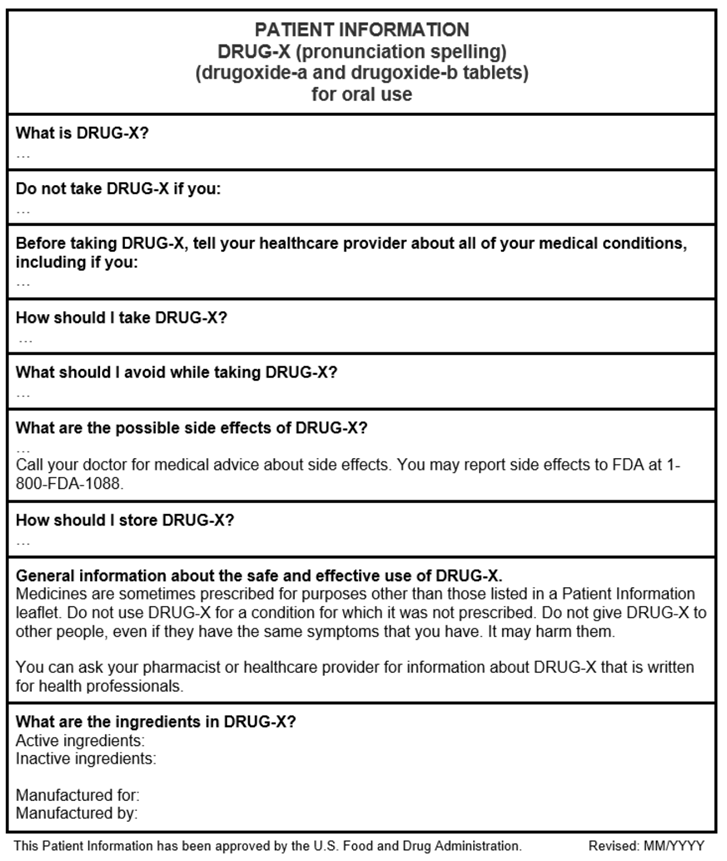
What are Patient Package Inserts?
A Patient Package Insert (PPI), also known as “Patient Information” is patient labeling that can be part of the FDA-approved prescription drug labeling. Certain PPIs are developed by the manufacturer and approved by the FDA.
PPIs are required for oral contraceptives and estrogen-containing products, and these PPIs are required to be dispensed with each prescription:
- PPIs for oral contraceptives (21 CFR 310.501)
- PPIs for estrogens (21 CFR 310.515)
PPIs for other prescription drugs are submitted to the FDA voluntarily by the manufacturer and approved by the FDA, but their distribution is not mandated.
A guidance that has specific recommendations for the PPI is the Child-Resistant Packaging Statements in Drug Product Labeling (final guidance).
Below see an example PPI template that provides a general overview of the PPI (this PPI does not contain any product-specific information). The headings in this example PPI may not be applicable to all PPIs. Consider using the enclosed Sample Template for Patient Package Inserts when developing a PPI (this sample template may not contain all the PPI requirements or guidance recommendations).
What are Instructions for Use?
The Instructions for Use (IFU) is patient labeling that can be part of FDA-approved prescription drug labeling for a biologics license application (BLA), a new drug application (NDA), or an abbreviated new drug application (ANDA). The IFU is developed by applicants for patients (or their caregivers) who use prescription drugs that have complicated or detailed patient-use instructions. The IFU provides detailed, action-oriented, step-by-step written and visual instructions for the patient on how to use the drug including instructions on preparation, administration, handling, storage, and disposal. The IFU is developed by the applicant, reviewed and approved by FDA, and provided to patients (or their caregivers) when the drug is dispensed. Some IFUs are not approved by the FDA. IFU resources include:
- Instructions for Use - Patient Labeling for Human Prescription Drug and Biological Products (final guidance)
- An additional guidance that has specific recommendations for the IFU: Metered Dose Inhaler and Dry Powder Inhaler Drug Products - Quality Considerations (draft guidance)
Below see an example IFU template that provides a general overview of the IFU (this IFU does not contain any product-specific information).
What is Patient Medication Information?
FDA published a proposed rule, Medication Guides: Patient Medication Information in May 2023. Under the proposed rule, FDA is proposing to amend its human prescription drug labeling regulations to require a new type of Medication Guide — Patient Medication Information (PMI) — for prescription drugs used, dispensed, or administered on an outpatient basis, as well as for blood and blood components transfused in an outpatient setting. When the rule is finalized, PMI will:
- Contain essential information that patients need to know about the prescription drug,
- Be an FDA-approved one-page document with standardized format and content requirements.
For additional information on the proposed PMI rule visit the Patient Medication Information webpage.
Contact Information
For specific application or supplement questions or for general questions about prescription drug labeling, please visit Prescription Drug Labeling Contact Information.
For general questions about patient labeling or this website please email CDEROMP@fda.hhs.gov.