Frequently Asked Questions about Labeling for Prescription Medicines
For Healthcare Professionals
Frequently asked questions about labeling for prescription drugs (medicines) on this webpage are primarily directed to healthcare professionals (for example, doctors, nurse practitioners, physician assistants, pharmacists, nurses). For information about prescription drug labeling resources primarily directed to industry such as those for the Prescribing Information, FDA-approved patient labeling, carton and container labeling, biological product labeling, generic drug labeling, labeling databases, and product databases visit FDA’s Labeling Resources for Prescription Drugs.
Labeling for prescription medicines is FDA’s primary tool for communicating drug information to healthcare professionals, and patients and their caregivers. Labeling for prescription medicines includes:
- Prescribing Information (labeling for healthcare professionals),
- Carton and container labeling (cartons and containers are outside packaging that contain information about prescription medicines), and
- Labeling for patients or caregivers (e.g., Medication Guides, Patient Package Inserts, and Instructions for Use).
Labeling for prescription medicines is required for all FDA-approved prescription medicines. Such labeling is:
- Proposed by the drug company,
- Reviewed by the FDA, and
- If acceptable, approved by the FDA. If the labeling for a new medicine is found to be unacceptable by the FDA, the medicine will not be approved by the FDA.
All prescription medicines have Prescribing Information and carton and/or container labeling and many, but not all, have labeling for patients or caregivers. For additional background about labeling for prescription medicines see:
- Frequently Asked Questions about Labeling for Prescription Medicines (2022 Presentation).
- Prescription Drug Labeling (2024 video)
- The Ins and Outs of Prescription Drug Labeling (2021 presentation)
The Prescribing Information is for healthcare professionals (e.g., doctors, nurse practitioners, physician assistants, pharmacists, nurses). The Prescribing Information contains a summary of the essential scientific information needed for the safe and effective use of the medicine. The most common type of Prescribing Information format is the Physician Labeling Rule (PLR) format which includes:
- Highlights of Prescribing Information
- Table of Contents, and
- Full Prescribing Information.
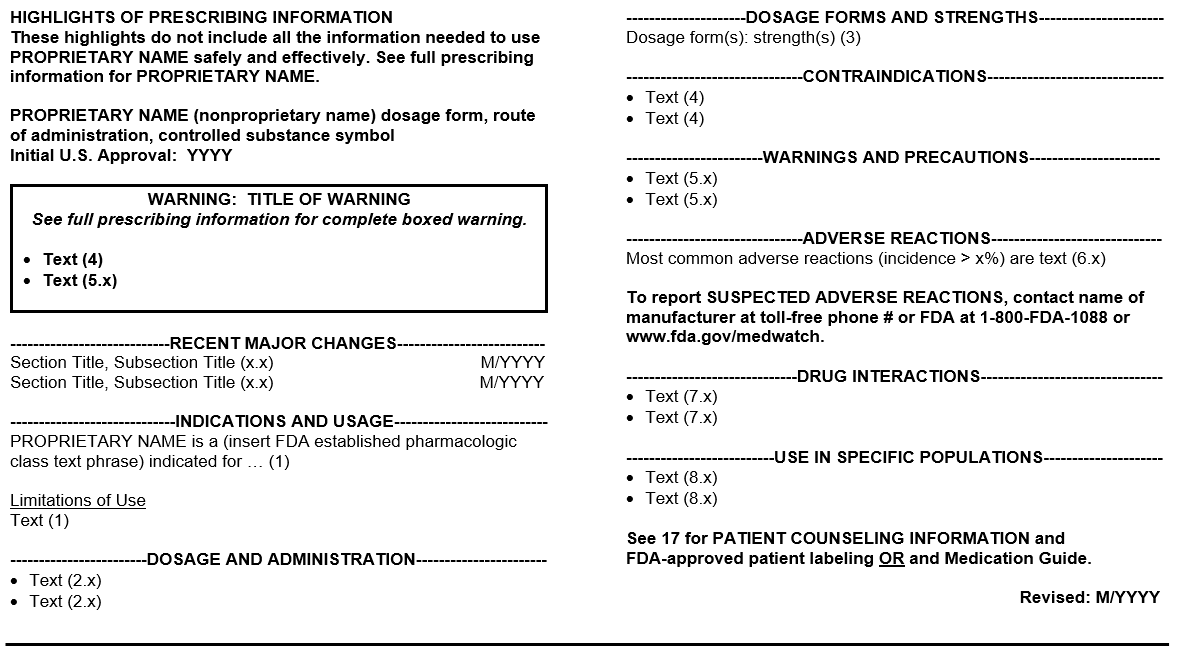
Highlights of Prescribing Information (referred to as Highlights) contain a concise summary of crucial prescribing information that the healthcare professional needs to use the medicine safely and effectively (Figure A includes the required and recommended format for Highlights).
Figure A: Highlights of Prescribing Information
Some key features of Highlights include:
- The Initial U.S. Approval is the four-digit year which FDA initially approved a new molecular entity, new biological product, or a new combination of active ingredients. For example, the Initial U.S. Approval for LIPITOR is 1996 because the new molecular entity (atorvastatin) was first approved in 1996.
- Recent Major Changes pertains to substantive changes to the BOXED WARNINGS, INDICATIONS AND USAGE, DOSAGE AND ADMINISTRATION, CONTRAINDICATIONS, or WARNINGS AND PRECAUTIONS sections. Each recent major changes listing includes the section or subsection heading and number and the month and year when the labeling change was included.
- The established pharmacologic class (EPC) in the Indications and Usage heading is a group of active moieties or substances that share scientifically valid properties associated with an approved indication. For example:
- “angiotensin converting enzyme inhibitor” is the EPC for lisinopril,
- “androgen” is the EPC for testosterone, and
- “aminoglycoside antibacterial” is the EPC for tobramycin
- The Adverse Reactions Contact Reporting Statement encourages reporting of suspected adverse events. This statement includes the phone number of the drug company responsible for collecting the information, and the phone number and webpage for the FDA to collect such information.
- The Revision Date is the month and year of the most recent revision to the Prescribing Information. The most recent revision may include:
- Significant updates to the Prescribing Information (e.g., adding a new indication and dosage), or
- Minor updates (e.g., correctly a typographical error or updating the address of the manufacturer).
- References in Highlights hyperlink to sections and subsections in the Full Prescribing Information when labeling is in an electronic format called Structured Product Labeling. For example, the “(1)” reference in the Highlights hyperlinks to the INDICATIONS AND USAGE section (Section 1) in the Full Prescribing Information.
The Table of Contents includes the sections (bolded) and subsections (indented in regular font) of the Full Prescribing Information in a two-column format (see Figure B for an example of the Table of Contents). Some formats of labeling include hyperlinks from the sections and subsections in the Table of Contents to the sections and subsections in the Full Prescribing Information.
Figure B: Table of Contents
The Full Prescribing Information includes a summary of the essential scientific information for the healthcare professional for the safe and effective use of the medicine (see Table C for a summary of the information in each of the sections of the Full Prescribing Information). The sections and many subsections in the Full Prescribing Information are consistently ordered and numbered. More commonly referenced information generally appears in the first several sections of the Full Prescribing Information.
Table C: Organization and Content of the Full Prescribing Information1
|
Section |
Type of Information Included |
|---|---|
|
Boxed Warning (sometimes referred to in lay terms as a “black-box warning”) |
|
|
1 Indications and Usage |
FDA-approved uses that are supported by substantial evidence of effectiveness, with benefits that outweigh risks |
|
2 Dosage and Administration |
|
|
3 Dosage Forms and Strengths |
Approved dosage forms, strengths, and identifying characteristics (e.g., shape, color, scoring, imprinting) |
|
4 Contraindications |
Situations when the risk from use clearly outweighs any possible therapeutic benefit, and the medicine must not be used |
|
5 Warnings and Precautions |
Description of clinically significant adverse reactions or risks with the medicine.
|
|
6 Adverse Reactions |
|
|
7 Drug Interactions |
Description of clinically significant drug interactions with other medicines, drug classes, or foods.
|
|
8 Use in Specific Populations |
Information on use of the medicine in:
|
|
9 Drug Abuse and Dependence |
Information on a medicine’s potential for abuse, misuse, addiction, dependence, and tolerance and about a medicine’s abuse-deterrent properties |
|
10 Overdosage |
Signs, symptoms, laboratory findings, and complications of overdosage and how to treat an overdosage |
|
11 Description |
Brand name, generic name or proper name, dosage form(s), route(s) of administration, and chemistry characteristics of the medicine (e.g., chemical and structural formula, a list of active and inactive ingredients in the medicine) |
|
12 Clinical Pharmacology |
|
|
13 Nonclinical Toxicology |
Information on the propensity of the medicine to cause cancers in animals, for mutagenesis, effects of the medicine on animal fertility, and other animal toxicology/pharmacology findings |
|
14 Clinical Studies |
Summary of the trial designs, baseline demographics and important disease characteristics, and results that established substantial evidence of effectiveness for all of the approved indications |
|
15 References |
Usually omitted, unless there are authoritative reference(s) that contain information not in the labeling that is important for the healthcare professional |
|
16 How Supplied/Storage and Handling |
Dosage forms, strengths, quantity of medicine available for prescribing (e.g., bottles of 100 tablets, 60-gram tubes), identifying characteristics including the National Drug Code (NDC) numbers, and special storage and handling conditions (e.g., store in refrigerator, protect from light) |
|
17 Patient Counseling Information |
Important information that the healthcare professional should convey to the patient or caregiver when a counseling decision is taking place (e.g., major risks of the medicine, critical administration instructions) |
1 If a section or information in a section is not pertinent to the specific medicine that section or information is not included in the Full Prescribing Information.
2 For the purposes of the Prescribing Information, an adverse reaction is an undesirable effect, reasonably associated with the use of a medicine, for which there is some basis to believe that there is a causal relationship between the medicine and the occurrence of the undesirable effect.
When the drug company submits a marketing application for their medicine, they must include data to support the safety and effectiveness of their medicine and their proposed Prescribing Information.
- Before Application Is Submitted
Drug companies submit a marketing application to the FDA to approve a new prescription medicine in the United States and/or to add new uses of an approved prescription medicine. Prior to submitting draft Prescribing Information to the FDA in a marketing application, the drug company may ask the FDA specific questions about their proposed Prescribing Information based on the studies they conducted or plan to conduct. The drug company may use FDA’s Labeling Resources for Prescription Drugs available to them.
- Drug Company Submits Draft Prescribing Information
When the drug company submits a marketing application for their medicine, they must include data to support the safety and effectiveness of their medicine and their proposed Prescribing Information.
- FDA Reviews Draft Prescribing Information
The FDA Prescribing Information review team for the marketing application conducts an active and ongoing review of the Prescribing Information from the time an application is received and throughout the review cycle. The review team identifies and addresses labeling issues that require resolution before the approval of the application.
- The FDA Prescribing Information review team typically consists of doctors with a specialty in the disease/condition being treated (e.g., endocrinologists review Prescribing Information for a medicine for diabetes), clinical pharmacology staff, labeling specialists, pharmacology/toxicology staff, product quality reviewers, promotional content specialists, regulatory project managers, safety experts (e.g., specialists in medication error prevention), statisticians, a cross-discipline team leader, and division and office management.
- Additional FDA reviewers with specific subject matter expertise are often included in the Prescribing Information review team based on the medicine’s proposed uses. For example, the following additional reviewers also review Prescribing Information: controlled substance specialists if the medicine is associated with abuse, misuse, addiction, dependence, or misuse; epidemiologists; engineers if the medicine is packaged or used with a device; microbiologists if the medicine is an antimicrobial; patient reported outcome specialists; pediatricians; and/or pharmacogenomic specialists. The FDA review team helps ensure that the Prescribing Information:
- Is scientifically accurate,
- Provides a summary of the essential scientific information needed for the safe and effective use of a medicine,
- Meets regulatory requirements,
- Is consistent with FDA guidance (as appropriate),
- Is informative and accurate, and
- Is neither promotional in tone nor false or misleading.
- FDA and Drug Company Discuss the Draft Prescribing Information
The FDA review team frequently asks the drug company for additional data to support or to clarify statements in the proposed Prescribing Information.
Final Prescribing Information development is an iterative process, typically involving several rounds of editing and discussions between FDA and the drug company to arrive at a final agreed-upon Prescribing Information (the final version of the Prescribing Information agreed upon by both the FDA and the drug company). If the FDA and the drug company cannot agree on the information in the Prescribing Information, the application will not be approved.
- Prescribing Information Approval and Communication
Upon approval of the application:
- FDA’s Center for Drug Evaluation and Research posts the approved Prescribing Information for prescription medicines and therapeutic proteins to Drugs@FDA
- FDA’s Center of Biologics Evaluation and Research posts approved Prescribing Information for vaccines, allergenic products, blood and blood products, plasma derivatives, and cellular and gene therapy products on CBER’s webpage.
Within 14 days of approval of the application (including the Prescribing Information), the drug company submits Structured Product Labeling to FDA’s electronic listing system. Subsequently electronic labeling is posted to several websites including FDA’s FDALabel and NIH’s DailyMed. See the 2017 presentation on the differences between labeling on Drugs@FDA versus DailyMed.
After initial approval, drug companies are required to update their Prescribing Information when new information becomes available that causes the Prescribing Information to be inaccurate, false, or misleading. Drug companies may submit supplements to their application and propose to include additional:
- Indications, uses, populations
- Dosages
- Safety information, or
- Other information on how to use the medicine safely and effectively.
The FDA may ask the drug company to voluntarily update their Prescribing Information by sending them a letter that asks them to submit a supplement to their application. The letter may ask the drug company to include safety information and/or to improve the communication of how to use the medicine safely and effectively in their Prescribing Information.
The FDA may require the company to update their Prescribing Information with safety labeling changes if the FDA becomes aware of new safety information (about a serious risk or an unexpected serious risk associated with the use of the medicine) that should be included in the Prescribing Information.
See examples of drugs that interact with cytochrome P-450 (CYP) enzymes or transporter systems.
Cartons and containers are the outside packaging that contain information about the prescription medicine. Carton and container labeling (for healthcare professionals, patients, or caregivers) contain key information such as the:
- Proprietary name - the brand name of the medicine that is sometimes referred to as the medicine’s trade name.
- For drug products, the nonproprietary name (sometimes referred to as the generic drug name)
- For biological products, the nonproprietary name (also known as the proper name) for
- Dosage form (e.g., tablets, oral solution)
- Strength
- Route(s) of administration. For example, topical (apply on top of skin), oral (take by mouth), and subcutaneous (inject directly under the skin).
- Warnings or cautionary statements (for example, “MYDRUG has multiple interactions with other drugs, consider the potential for these drug interactions prior to and during treatment with MYDRUG”), and
- For controlled substances, the controlled substance symbol.
Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to reduce the risk of medication errors.
FDA-approved labeling for prescription medicines for patients and caregivers include:
- Medication Guides,
- Patient Package Inserts, and
- Instructions for Use
Not all prescription medicines have FDA-approved Medication Guides, Patient Package Inserts, or Instructions for Use.
Consumer medication information (CMI) is written information for patients and caregivers about a prescription medicine that is developed by an individual(s) or organization other than the drug company. FDA does not review or approve CMI, and the drug company does not review CMI. CMI is intended to be given to patients or caregivers at the time they receive a prescription.
If you have questions about your drug (medicine), talk with your healthcare professional (for example, your doctor or pharmacist).
Medication Guides are a type of labeling for patients and caregivers that are required by the FDA when:
- The Medication Guide could help prevent serious side effects,
- The medicine has serious side effects in which patients should be made aware of, or
- Following directions for the use of the medicine is important for the medicine’s effectiveness.
Medication Guides are developed by the drug company and reviewed and approved by FDA.
Medication Guides include information about the medicine, how to take the medicine, who should not take the medicine, serious side effects of the medicine, and common side effects of the medicine. A Medication Guide must be provided to the patient whenever the medicine is dispensed.
For more information about Medication Guides, see the Patient Labeling Resources website for industry.
Patient Package Inserts are also known as “Patient Information” and are a type of labeling for patients and caregivers. Patient Package Inserts include information about the medicine, how to take the medicine, who should not take the medicine, serious side effects of the medicine, and common side effects of the medicine.
Patient Package Inserts are required for contraceptives given orally and for medicines that have estrogen. These Patient Package Inserts are developed by the drug company and reviewed and approved by FDA. Patient Package Inserts for these specific products must be given to each patient or their caregiver whenever the medicine is dispensed.
Patient Package Inserts for other medicines may be voluntarily submitted by the drug company to the FDA on their initiative or by FDA request. These Patient Package Inserts are approved by the FDA. These Patient Package Inserts are not required to be given to each patient.
For more information about Patient Package Inserts, see the Patient Labeling Resources website for industry.
Instructions for Use are a type of labeling for patients and caregivers for prescription medicines that have complicated or detailed instructions about use of the medicine. For example, medicines administered by an autoinjector, a transdermal system (commonly known as a patch), or a syringe, or medicines that require mixing before use. Instructions for Use provides detailed, step-by-step written and visual instructions for the patient or caregiver on how to:
- Use the medicine,
- Prepare the medicine,
- Administer the medicine,
- Handle the medicine,
- Store the medicine, and
- Throw away (dispose of) the medicine.
Instructions for Use is developed by the drug company, and reviewed and approved by FDA, and provided to patients (or their caregivers) when the medicine is dispensed. Some IFUs are not approved by the FDA.
For more information about Instructions for Use, see the Patient Labeling Resources website for industry.
For labeling, scientific, regulatory, and clinical considerations about biosimilars, see the presentation.
Contact Information
For specific application or supplement questions or for general questions about prescription drug labeling, please visit Prescription Drug Labeling Contact Information.