Drug Trials Snapshots: IMCIVREE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to IMCIVREE Prescribing Information for complete information.
IMCIVREE (setmelanotide)
im-SIV-ree
RHYTHM Pharmaceuticals, Inc.
Approval date: November 25, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
IMCIVREE is a drug used for long-lasting weight management in patients 6 years and older who are obese because of a specific enzyme deficiency [(proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency)].
How is this drug used?
IMCIVREE is an injection given under the skin (subcutaneously) once daily. The daily dose is based on patient’s weight.
What are the benefits of this drug?
After one-year treatment with IMCIVREE, 80% of patients with POMC or PCSK1 deficiency and 46% of patients with LEPR deficiency achieved at least 10% weight loss.
What are the benefits of this drug (results of trials used to assess efficacy)?
The primary endpoint for both trials was achieving a ≥10% weight loss after 1 year of treatment with IMCIVREE.
Table 1. Body Weight (kg) – Proportion of Patients Achieving at Least 10% Weight Loss from Baseline at 1 Year in Trial 1 and Trial 2
|
Parameter |
Statistic |
Trial 1 |
Trial 2 (LEPR) |
|
Patients Achieving at Least 10% Weight Loss at Year 1 |
n (%) |
8 (80%) |
5 (46%) |
|
95% CI1 |
(44.4%, 97.5%) |
(16.8%, 76.6%) |
|
|
P-value2 |
<0.0001 |
0.0002 |
|
|
Note: The analysis set includes patients who received at least 1 dose of study drug and had at least 1 baseline assessment. |
|||
IMCIVREE Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
It was not possible to determine differences in how well IMCIVREE worked among sex, race, and age groups because of the small number of patients in each group.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup analyses for individual trials based on the primary endpoint defined as percentage of patients, achieving ≥10% weight loss at 1 year are presented below. Given the small sample sizes for the subgroups, results must be interpreted with caution.
Table 2. Subgroup Analyses of the Primary Endpoint
|
|
Trial 1 (N=10) |
Trial 2 (N=11)1 |
|
Sex |
||
|
Female, % (n/N) |
80 (4/5) |
25 (2/8) |
|
Male, % (n/N) |
80 (4/5) |
100 (3/3) |
|
Race |
||
|
White, % (n/N) |
86 (6/7) |
50 (5/10) |
|
All Other, % (n/N) |
67 (2/3) |
0 (0/1) |
|
Age |
|
|
|
<18years, %(n/N) |
83 (5/6) |
33 (1/3) |
|
≥18 years, % (n/N) |
75 (3/4) |
50 (4/8) |
1Two patients had missing values for body weight at 1 year: one had an adverse event and was considered a non-responder; the other was considered responder based on linear extrapolation.
FDA Statistical Review
What are the possible side effects?
IMCIVREE may cause sexual dysfunction, depression, suicidal thoughts and skin darkening.
The most common side effects are injection site reactions, skin darkening, nausea and headache.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in patients treated with IMCIVREE.
Table 3. Adverse Reactions Occurring in 3 or More Patients Treated with IMCIVREE in Open-Label Clinical Trials of 52-weeks Duration
|
|
IMCIVREE-treated Patients |
|
Injection site reaction a |
96 |
|
Skin hyperpigmentation b |
78 |
|
Nausea |
56 |
|
Headache |
41 |
|
Diarrhea |
37 |
|
Abdominal pain c |
33 |
|
Back pain |
33 |
|
Fatigue |
30 |
|
Vomiting |
30 |
|
Depression d |
26 |
|
Upper respiratory tract infection |
26 |
|
Spontaneous penile erection e |
23 |
|
Arthralgia |
19 |
|
Asthenia |
19 |
|
Dizziness |
15 |
|
Dry mouth |
15 |
|
Dry skin |
15 |
|
Insomnia |
15 |
|
Vertigo |
15 |
|
Alopecia |
11 |
|
Chills |
11 |
|
Constipation |
11 |
|
Influenza-like illness |
11 |
|
Muscle spasm |
11 |
|
Pain in extremity |
11 |
|
Rash |
11 |
|
Suicidal ideation |
11 |
a Includes injection site erythema, pruritus, edema, pain, induration, bruising, hypersensitivity, hematoma, nodule, and discoloration
b Includes skin hyperpigmentation, pigmentation disorders, skin discoloration
c Includes abdominal pain and upper abdominal pain
d Includes depressed mood
e n = 13 male patients
IMCIVREE Prescribing Information
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The majority of patients were White; therefore, differences in side effects among different races could not be determined.
- Age: The majority of patients were 12 years and older; therefore, differences in the occurrence of side effects among different age groups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Summaries of adverse reactions from both trials by sex and age are presented below.
Table 4. Adverse Reactions by Sex
|
|
Male (N=13) |
Female (N= 14) |
|
Injection site reaction a |
92 |
100 |
|
Skin hyperpigmentation b |
69 |
86 |
|
Nausea |
30 |
50 |
Table 5. Adverse Reactions by Age Group
|
|
<12 years (N=4) |
≥12 years (N=23) |
|
Injection site reaction a |
75 |
100 |
|
Skin hyperpigmentation b |
100 |
74 |
|
Nausea |
25 |
61 |
a Includes injection site erythema, pruritus, edema, pain, induration, bruising, hypersensitivity, hematoma, nodule, and discoloration
b Includes skin hyperpigmentation, pigmentation disorders, skin discoloration
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
There were two clinical trials (Trial 1 /NCT02896192) and Trial 2/ NCT03287960) that enrolled 27 patients with obesity due to specific genetic errors. The trials were conducted at 13 centers in the United Kingdom, the United States, France, Germany, Netherland, Canada, Spain, and Belgium.
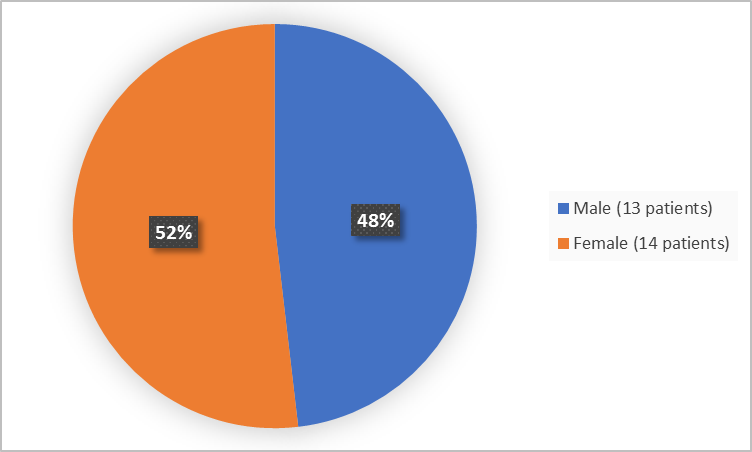
The figure below summarizes patients by sex who were in Trials 1 and 2.
Figure 1. Baseline Demographics by Sex (safety population-Trials 1 and 2)
Clinical Trial Data
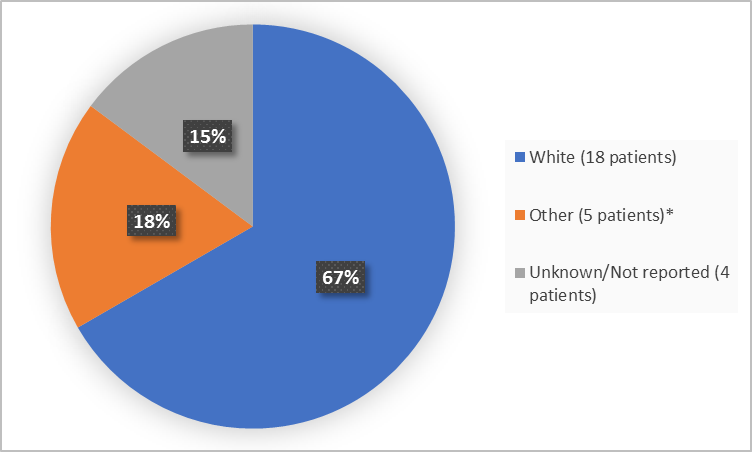
The figure below summarizes patients by race who were in Trials 1 and 2.
Figure 2. Baseline Demographics by Race (safety population-Trials 1 and 2)
*includes Asian, Arab, Turkish and Moroccan
Clinical Trial Data
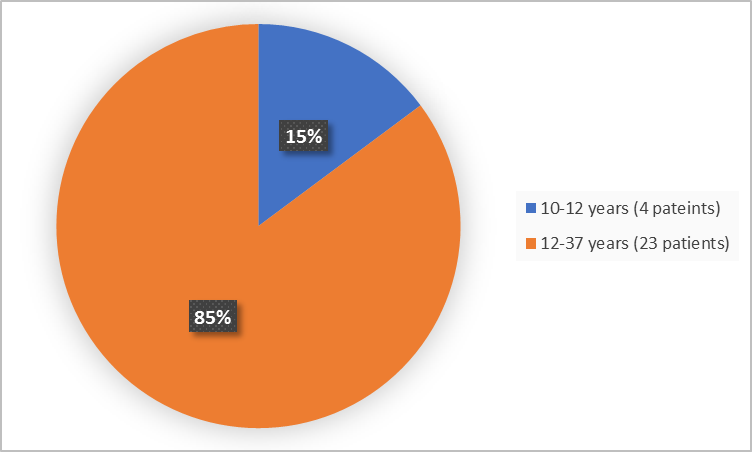
Figure 3 summarizes the percentage of patients by age group in the Trials 1 and 2.
Figure 3. Baseline Demographics by Age (safety population-Trials 1 and 2)
Clinical Trial Data
Figure 4 summarizes the percentage of patients by ethnicity in the Trials 1 and 2.
Figure 4. Baseline Demographics by Ethnicity (safety population-Trials 1 and 2)
Clinical Trial Data
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials.
Table 6. Demographics of Patients in the Clinical Trials (safety population)
|
Parameter |
Trial 1 |
Trial 2 |
TOTAL |
|
Sex, n (%) |
|
|
|
|
Male |
8 (57) |
5 (38) |
13 (48) |
|
Female |
6 (43) |
8 (62) |
14 (52) |
|
Race, n (%) |
|
|
|
|
White |
8 (57) |
10 (77) |
18 (67) |
|
Other |
6 (43) |
3 (23) |
5 (19) |
|
Asian |
0 |
1 (8) |
1 (4) |
|
Arab |
1 (7) |
0 |
1 (4) |
|
Turkish |
2 (14) |
0 |
2 (7) |
|
Moroccan |
1 (7) |
0 |
1 (4) |
|
Unknown |
1 (7) |
2 (15) |
3 (11) |
|
Not Reported |
1 (7) |
0 |
1 (4) |
|
Age at Enrollment (years) |
|
|
|
|
Median |
16.5 |
23.0 |
20 |
|
Min, Max |
10, 30 |
13, 37 |
10, 37 |
|
Age Categories, n (%) |
|
|
|
|
< 12 years |
4 (29) |
0 |
4 (15) |
|
≥ 12 years |
10 (71) |
13 (100) |
23 (85) |
|
Ethnicity, n (%) |
|
|
|
|
Hispanic or Latino |
2 (14) |
0 |
2 (7) |
|
Not Hispanic or Latino |
10 (71) |
11 (85) |
21 (78) |
|
Unknown |
2 (14) |
2 (15) |
4 (15) |
|
Country, n (%) |
|
|
|
|
United Kingdom |
0 |
1 (8) |
1 (4) |
|
United States |
1 (7) |
0 |
1 (4) |
|
France |
2 (14) |
6 (46) |
8 (30) |
|
Germany |
7 (50) |
3 (23) |
10 (37) |
|
Netherlands |
0 |
3 (23) |
3 (11) |
|
Canada |
1 (7) |
0 |
1 (4) |
|
Spain |
1 (7) |
0 |
1 (4) |
|
Belgium |
2 (14) |
0 |
2 (7) |
Clinical Trial Data
How were the trials designed?
There were two identical trials that evaluated the benefit and side effects of IMCIVREE.
In both trials, patients had genetic testing performed to confirm that obesity was related to the lack of the specific enzyme. All patients received IMCIVREE once daily according to their weight.
The benefit of IMCIVREE was measured by the percentage of treated patients who achieved at least 10% weight loss after one year of treatment.
How were the trials designed?
The safety and efficacy of IMCIVREE were evaluated in two identical 1-year, open-label trials, each with an 8-week, double-blind withdrawal period. Patients were 6 years and older and had genetically confirmed or suspected POMC or PCSK1 deficiency obesity (Trial 1), or genetically confirmed or suspected LEPR deficiency obesity (Trial 2). Dose titration occurred over a 2 to 12-week period, followed by a 10-week, open-label treatment period. Patients who achieved at least a 5 kg weight loss (or at least 5% weight loss if baseline body weight was <100 kg) at the end of the open-label treatment period continued into a double-blind withdrawal period lasting 8 weeks, including 4 weeks of IMCIVREE followed by 4 weeks of placebo (investigators and patients were blinded to this sequence). Following the withdrawal sequence, patients re-initiated active treatment with IMCIVREE at the therapeutic dose for up to 32 weeks.
The endpoint for both trials was achieving a ≥10% weight loss after 1 year of treatment with IMCIVREE.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.