Drug Trial Snapshot: MEPSEVII
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the MEPSEVII Package Insert for complete information.
MEPSEVII (vestronidase alfa-vjbk)
mep-SEV-ee
Ultragenyx Pharmaceuticals Inc.
Approval date: November 15, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MEPSEVII is used to treat children and adult patients with mucopolysaccharidosis VII (also known as MPS VII or Sly Syndrome).
Mucopolysaccharidosis VII is a rare, inherited disease. It is caused by a low level of an enzyme (β-glucuronidase). The lack of an enzyme causes mucopolysaccharides (long chains of sugar molecules) to build up in the tissues and organs of the body, causing progressive damage.
MEPSEVII is an enzyme replacement therapy that works by replacing the missing enzyme.
How is this drug used?
MEPSEVII is given by a healthcare provider directly, into the bloodstream, through a needle in the vein, intravenous (IV) infusion every two weeks. MEPSEVII is infused over 4 hours.
The amount of drug used depends on patient’s weight.
What are the benefits of this drug?
After 24 weeks of treatment, patients who received MEPSEVII walked a longer distance (about 18 meters) in comparison to placebo treatment during the 6-minute walk test (6MWT).
Two additional patients in the MEPSEVII development program experienced marked improvement in lung function.
The table below summarizes results of the difference in the 6MWT when comparing MEPSEVII to placebo.
Table 2. Mean Difference in 6MWT (meters) Between MEPSEVII and Placebo Treatment in Patients with MPS VII
| Duration of MEPSEVII Treatment | LS mean 6MWT (meters) (± Standard Error)* |
Number and Treatment Assignment of Patients Included in Analysis** |
|---|---|---|
| 8 weeks | -11 (± 24) | 5 placebo period; 8 MEPSEVII period |
| 16 weeks | 13 (± 32) | 5 placebo period; 8 MEPSEVII period |
| 24 weeks | 18 (± 33) | 5 placebo period; 8 MEPSEVII period |
*ANCOVA analysis of change from baseline in least squares (LS) mean between placebo and MEPSEVII for different periods, after adjusting for study cohort, age, and baseline 6MWT distance. Patients who used assistive devices were imputed as zeros in the analysis. **Number and treatment assignment of patients included in the analysis was based upon a randomized start trial design and patient ability to complete testing. Due to no placebo period for the three patients who received 48 weeks of MEPSEVII in the first cohort of the randomized start design, more data were available for analyses during the treatment period (n=8) than during the placebo period (n=5). While data from 8 participants were available at each time point, they were not the same 8 participants due to missing observations.
Additional follow-up for up to 120 weeks suggested continued improvement in three patients and stabilization in seven others.
Two additional patients in the MEPSEVII development program (NCT01856218) experienced marked improvement in pulmonary function.
MEPSEVII Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The number of patients in the trial was too small (12 patients) to determine differences in sex, race, and age subgroups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
There were insufficient numbers of patients to perform any subgroup analyses.
What are the possible side effects?
MEPSEVII may cause a serious allergic reaction (anaphylaxis). Anaphylaxis may include difficulty breathing, low blood pressure, and slow heartbeat.
The most common side effects of MEPSEVII are leakage of drug from the vein into surrounding tissue, diarrhea, rash, swelling in arms and legs, itching, severe allergic reaction(anaphylaxis), infusion site swelling, and headache.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in patients treated with MEPSEVII.
Table 3. Adverse Reactions in Patients with MPSEVII
| Adverse Reaction | MEPSEVII N =12 n (Incidence Rate*) |
Placebo N=9 n (Incidence Rate*) |
|---|---|---|
| Infusion site extravasation | 4 (0.5) | 1 (0.4) |
| Diarrhea | 3 (0.4) | 0 (0.0) |
| Rash | 3 (0.4) | 2 (0.7) |
| Anaphylaxis | 2 (0.2) | 0 (0.0) |
| Infusion site swelling | 1 (0.1) | 0 (0.0) |
| Peripheral swelling | 1 (0.1) | 0 (0.0) |
| Pruritus | 1 (0.1) | 0 (0.0) |
n = number of reactions
*Adverse reaction incidence rates calculated per 8.3 patient years for exposure to MEPSEVII, and 2.7 years of exposure for placebo
MEPSEVII Prescribing Information
Were there any differences in side effects among sex, race and age?
The number of patients in the trial was too small (12 patients) to determine differences in sex, race, and age subgroups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
There were insufficient numbers of patients to perform any subgroup analyses.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved MEPSEVII based primarily on evidence from one clinical trial (NCT02230566) of 12 patients with mucopolysaccharidosis VII. The trial was conducted at 4 sites in the United States.
Figure 1 summarizes how many patients were enrolled in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 9 | 75% |
| Other/Unknown | 3 | 25% |
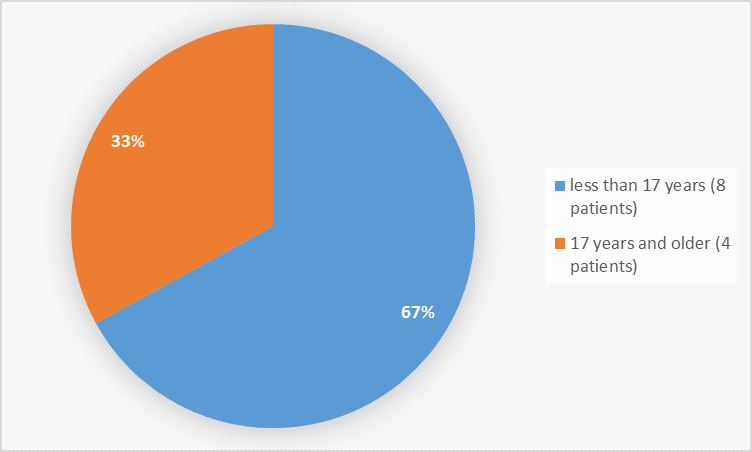
Figure 3 summarizes the percentage of patients by age group in the trial.
Figure 3. Baseline Demographics by Age
FDA Review
The table below summarizes demographics of patients in the clinical trial.
Table 4. Baseline Demographics
| Demographic Parameters | Trial 1 | Total (N=12) n (%) |
|||
|---|---|---|---|---|---|
| Study Arm A Placebo:0 weeks MEPSEVII:48 weeks (N=3) |
Study Arm B Placebo:8 weeks MEPSEVII:40 weeks (N=3) |
Study Arm C Placebo:16 weeks MEPSEVII:32 weeks (N=3) |
Study Arm D Placebo:24 weeks MEPSEVII:24 weeks (N=3) |
||
| Sex | |||||
| Male, n (%) | - | 1 (33) | - | 3 (100) | 4 (33) |
| Female, n (%) | 3 (100) | 2 (67) | 3 (100) | - | 8 (67) |
| Age | |||||
| Mean years (SD) | 13.2 (1.7) | 12.5 (4.0) | 20.8 (3.4) | 15.2 (8.6) | 15.4 (5.5) |
| Median (years) | 13.4 | 12.8 | 22.5 | 10.5 | 14.1 |
| Min, max (years) | 11.5,14.7 | 8.4,16.4 | 17.3,22.6 | 10.1,25.2 | 8.5, 25.2 |
| Age Group | |||||
| < 17="" years,="" n=""> | 3 (100) | 3 (100) | 2 (67) | 8 (67) | |
| ≥ 17 - < 65="" years,="" n=""> | 3 (100) | 1 (33) | 4 (33) | ||
| Race | |||||
| White, n (%) | 3 (100) | 2 (67) | 2 (67) | 2 (67) | 9 (75) |
| Other, n (%) | 1 (33) | 1 (33) | 1 (33) | 3 (25) | |
| Ethnicity | |||||
| Hispanic or Latino, n (%) | 2 (67) | 2 (67) | 1 (33) | 1 (33) | 6 (50) |
| Not Hispanic or Latino, n (%) | 1 (33) | 1 (33) | 2 (67) | 2 (67) | 6 (50) |
Adapted from FDA Review
How were the trials designed?
The benefit and side effects of MEPSEVII were based primarily on one trial. Patients were randomly assigned to four groups. Three groups of patients received placebo treatment before starting MEPSEVII treatment and one group received MEPSEVII only. MEPSEVII or placebo were given once every two weeks as intravenous (IV) infusions. Neither patients nor healthcare providers knew which treatment was given until after the trial was competed.
The benefit of 24 weeks of MEPSEVII treatment was primarily evaluated by the 6-minute walking test (6MWT) and compared to placebo treatment in ten patients who could perform the test. The 6MWT measured the distance a patient could walk on a flat surface in 6 minutes. An additional follow-up using 6MWT was done for up to 120 weeks.
How were the trials designed?
The clinical trial was a randomized start multicenter trial in patients with mucopolysaccharidosis VII.
Patients were randomized into one of four study arms and received MEPSEVII administered as a 4-hour infusion every 2 weeks for 24 weeks of treatment. Patients, in 3 study arms, received placebo prior to starting treatment with MEEPSEVII but the timing of cross-over was blinded.
Efficacy was primarily assessed via the six-minute walk test (6 MWT) in ten patients who could perform the test before and following 24 weeks of MEPSEVII treatment with an additional follow-up for up to 120 weeks.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION
Adapted from FDA Review