More Information for ENTYVIO (vedolizumab) To Treat Crohn’s Disease
Who participated in the study?
The FDA approved ENTYVIO based on evidence from three trials in subjects with moderate to severe Crohn’s Disease conducted in North America, Europe, Asia, Africa and Australia. The baseline demographics by subgroup included in CD Trials I, II, and III are summarized in Tables 3, 4, and 5, respectively.
Table 3: Demographics of Efficacy Population – CD Trial I
| Induction Cohort 1 ITT Population | ||
|---|---|---|
| PLACEBO (N=148) | ENTYVIO (N=220) | |
| Gender, n ( % ) | ||
| Male | 69 (47) | 105 (48) |
| Female | 79 (53) | 115 (52) |
| Race, n (%) | ||
| White | 124 (84) | 182 (83) |
| Black | 3 (2) | 3 (1) |
| Asian | 19 (13) | 35 (16) |
| Other | 2 (1) | 0 |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 5(3) | 2 (<> |
| Not Hispanic or Latino | 139 (94) | 214 (97) |
| Not reported | 4 (3) | 4 (2) |
| Age (yrs), n (%) | ||
| Mean (Std Dev) | 38.6 (13.16) | 36.3 (11.57) |
| Median | 36.7 | 34.8 |
| Minimum, maximum | 19, 75 | 18, 77 |
| Age (yrs ), n ( % ) | ||
| <> | 67 (45) | 111 (50) |
| ≥ 35 | 81 (55) | 109 (50) |
| Age (yrs ), n ( % ) | ||
| <> | 142 (96) | 218 (99) |
| ≥ 65 | 6 (4) | 2(<> |
Source: Adapted from Statistical Review, Table 1. p 111
Table 4: Demographics of Efficacy Population – CD Trial II
|
|
Overall ITT Population | ||
|---|---|---|---|
|
|
PLACEBO N=207 | VDZ (ENTYVIO) N=209 | TOTAL N=416 |
| Gender, n (%) | |||
| Male | 89 (43) | 91 (44) | 180 (43) |
| Female | 118 (57) | 118 (56) | 236 (57) |
| Race, n (%) | |||
| White | 186 (90) | 188 (90) | 374 (90) |
| Black | 5 (2) | 4 ( 2 ) | 9 (2) |
| Asian | 9 (4) | 9 (4) | 18 (4) |
| Other | 7 (3) | 6 (3) | 13 (3) |
| Not reported | 0 | (<> | (<> |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 4 (2) | 4 ( 2 ) | 8 ( 2 ) |
| Not Hispanic or Latino | 199 (96) | 204 (98) | 403 (97) |
| Not reported | 4 (2) | 1 (<> | 5 (1) |
| Age (yrs), n (%) | |||
| Mean (Std Dev) | 37.1 (13.15) | 38.6 (12.14) | 37.9 (12.66) |
| Median | 34.8 | 36.9 | 36.2 |
| Min, Max | 19, 77 | 20, 69 | 19, 77 |
| Age (yrs), n (%) | |||
| <> | 105 (51) | 88 (42) | 193 (46) |
| ≥35 | 102 (49) | 121 (58) | 223 (54) |
| Age (yrs), n (%) | |||
| <> | 202 (98) | 206 (99) | 408 (98) |
| ≥65 | 5 (2) | 3 (1) | 8 (2) |
Source: Adapted from Statistical Review, Table 24. p 140
Table 5: Demographics of Efficacy Population – CD Trial III
|
|
Maintenance ITT | ||
|---|---|---|---|
|
|
[Responders to VDZ (ENTYVIO) induction, randomized to Maint. Treatment at Week 6] | ||
|
|
PLACEBO N = 153 |
VDZ Q8W N = 154 |
VDZ Q4W N = 154 |
| Gender, n (%) | |||
| Male | 72 (47) | 68 (44) | 82 (53) |
| Female | 81 (53) | 86 (56) | 72 (47) |
| Race, n (%) | |||
| White | 140 (92) | 136 (88) | 134 (87) |
| Black | 4 (3) | 4 (3) | 2 ( 1 ) |
| Asian | 9 (6) | 14 (9) | 15 (10) |
| Other | 0 | 0 | 3 (2) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 2 ( 1 ) | 3 (2) | 2 ( 1) |
| Not Hispanic or Latino | 148 (97) | 148 (96) | 149 (97) |
| Not reported | 3 (2) | 3 (2) | 3 (2) |
| Age (yrs) | |||
| Mean (Std Dev) | 37.2 (11.95) | 35.1 (12.23) | 34.9 (12.20) |
| Median | 36 | 32.5 | 32.7 |
| Minimum, maximum | 18, 68 | 18, 72 | 19, 77 |
| Age (yrs ), n (%) | |||
| <> | 73 (48) | 89 (58) | 84 (55) |
| ≥35 | 80 (52) | 65 (42) | 70 (45) |
| Age (yrs), n (%) | |||
| <> | 149 (97) | 151 (98) | 152 (99) |
| ≥65 | 4(3) | 3 (2) | 2 (1) |
Source: Adapted from Statistical Review, Table 12. p 125
The baseline demographics by subgroup in the UC/CD Combined safety population is shown in Table 6.
Table 6: Demographics of UC/CD Comparative Safety Population
|
|
Overall ITT Population | ||
|---|---|---|---|
|
|
ITT PLACEBO N=297 |
Non-ITT PLACEBO N=297 |
Combined ENTYVIO N=1434 |
| Gender, n (%) | |||
| Male | 141 (51) | 161 (54) | 743 (52) |
| Female | 138 (49) | 136 (46) | 691 (48) |
| Race , n (%) | |||
| White | 241 (86) | 239 (80) | 1249 (87) |
| Black | 6 (2) | 5 (2) | 24 (2) |
| Asian | 29 (10) | 51 (17) | 144 (10) |
| Other | 3 (1) | 2 (<> | 17 (1) |
| Age (yrs) | |||
| Mean (Std Dev) | 38.6 (12.94) | 39.9 (12.88) | 37.5 (12.62) |
| Median | 36.7 | 38.9 | 35.6 |
| Min, Max | 18, 74 | 19, 76 | 18, 78 |
| Age in years, n (%) | |||
| <> | 270 (97) | 284 (96) | 1400 (98) |
| ≥65 | 9 (3) | 13 (4) | 34 (2) |
How was the study designed?
The safety and efficacy of ENTYVIO were evaluated in three randomized, double-blind, placebo-controlled clinical trials (CD Trials I, II, and III) in adult patients with moderately to severely active Crohn’s disease (CD) (Crohn’s Disease Activity Index [CDAI] score of 220 to 450). Enrolled patients in the United States (US) had over the previous five-year period an inadequate response or intolerance to immunomodulator therapy (i.e., azathioprine, 6 mercaptopurine, or methotrexate) and/or an inadequate response, loss of response, or intolerance to one or more TNF blockers. Outside the US, prior treatment with corticosteroids was sufficient for entry if over the previous five-year period the patients were corticosteroid dependent (i.e., unable to successfully taper corticosteroids without a return of the symptoms of CD) or had an inadequate response or intolerance to corticosteroids.
CD Trial I (Study Design)
In CD Trial I, 368 patients were randomized in a double-blind fashion (3:2) to receive ENTYVIO 300 mg or placebo by intravenous infusion at Week 0 and Week 2. Efficacy assessments were at Week 6. Concomitant stable dosages of aminosalicylates, corticosteroids (prednisone dosage ≤30 mg/day or equivalent), and immunomodulators (azathioprine, 6mercaptopurine or methotrexate) were permitted through Week 6.
CD Trial II (Study Design)
Compared to CD Trial I, CD Trial II enrolled a higher number of patients who had over the previous five-year period had an inadequate response, loss of response, or intolerance to one or more TNF blockers (76%); this was the primary analysis population. In CD Trial II, 416 patients were randomized in a double-blind fashion (1:1) to receive either ENTYVIO 300 mg or placebo at Weeks 0, 2 and 6. Efficacy assessments were at Weeks 6 and 10. Concomitant aminosalicylates, corticosteroids, and immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) were permitted through Week 10.
CD Trial III (Study Design)
In order to be randomized to treatment in CD Trial III, patients had to have received ENTYVIO and be in clinical response (defined as a ≥70-point decrease in CDAI score from baseline) at Week 6. Patients could have come from either CD Trial I or from a group who received ENTYVIO open-label.
In CD Trial III, 461 patients were randomized in a double-blind fashion (1:1:1) to one of the following regimens beginning at Week 6: ENTYVIO 300 mg every eight weeks, ENTYVIO 300 mg every four weeks or placebo every four weeks. Efficacy assessments were at Week 52. Concomitant aminosalicylates and corticosteroids were permitted through Week 52. Concomitant immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) were permitted outside the US but were not permitted beyond Week 6 in the US.
What are the results of the efficacy study?
CD Trial I (Results)
At baseline, patients were receiving corticosteroids (49%), immunomodulators (azathioprine, 6 mercaptopurine, or methotrexate) (35%), and/or aminosalicylates (46%). Forty-eight percent of the patients had an inadequate response, loss of response, or intolerance to a TNF blocker therapy. Seventeen percent of patients had inadequate response, inability to taper, or intolerance to prior corticosteroid treatment only (i.e., had not received prior immunomodulators or TNF blockers). The median baseline CDAI score was 324 in the ENTYVIO group and 319 in the placebo group.
In CD Trial I, a statistically significantly higher percentage of patients treated with ENTYVIO achieved clinical remission (defined as CDAI ≤150) as compared to placebo at Week 6 (Table 7). The difference in the percentage of patients who demonstrated clinical response (defined as a ≥100-point decrease in CDAI score from baseline), was however, not statistically significant at Week 6.
CD Trial II (Results)
At baseline, patients were receiving corticosteroids (54%), immunomodulators (azathioprine, 6mercaptopurine, or methotrexate) (34%), and aminosalicylates (31%). The median baseline CDAI score was 317 in the ENTYVIO group and 301 in the placebo group.
For the primary endpoint (clinical remission at Week 6), treatment with ENTYVIO did not result in statistically significant improvement over placebo (Table 7). Secondary endpoints including assessments at Week 10 were not tested because the primary endpoint was not statistically significant.
Table 7. Proportion of Patients in Clinical Remission at Week 6 (CD Trials I and II)
| Placebo | ENTYVIO | p-value | Treatment Difference and 95% CI |
|
|---|---|---|---|---|
| CD Trial I: Clinical Remission* at Week 6 |
7% (10/148) | 15% (32/220) | ‡ 0.041 |
8% (1%, 14%) |
| CD Trial II†: Clinical Remission* at Week 6 |
12% (19/157) | 15% (24/158) | § NS |
3% (-5%, 11%) |
*Clinical Remission: CDAI ≤150
†The primary analysis population for CD Trial II was patients that had an inadequate response, loss of response, or intolerance to one or more TNF blockers (76% of the overall population)
‡Adjusted p-value for multiple comparisons of two primary endpoints
§NS: Not significant (Secondary endpoints including assessments at Week 10 were not tested because the CD Trial II primary endpoint was not statistically significant)
Source: Drug Label, Table 5
A statistically significantly higher percentage of patients treated with ENTYVIO achieved clinical remission (defined as CDAI ≤150) as compared to placebo at Week 6. For the primary endpoint (clinical remission at Week 6), treatment with ENTYVIO did not result in statistically significant improvement over placebo)
CD Trial III (Results)
At Week 6, patients were receiving corticosteroids (59%), immunomodulators (azathioprine, 6 mercaptopurine, or methotrexate) (31%), and aminosalicylates (41%). Fifty-one percent of patients had an inadequate response, loss of response, or intolerance to a TNF blocker therapy. At Week 6, the median CDAI score was 322 in the ENTYVIO every eight week group, 316 in the ENTYVIO every four week group, and 315 in the placebo group. Patients who had achieved clinical response (≥70 decrease in CDAI score from baseline) at Week 6 and were receiving corticosteroids were required to begin a corticosteroid-tapering regimen at Week 6.
In CD Trial III, a greater percentage of patients in groups treated with ENTYVIO as compared to placebo were in clinical remission (defined as CDAI score ≤150) at Week 52. A greater percentage of patients in groups treated with ENTYVIO as compared to placebo had a clinical response (defined as ≥100 decrease in CDAI score from baseline) at Week 52 (Table 8). In the subgroup of patients who were receiving corticosteroids at baseline and who were in clinical response at Week 6 (defined as ≥70 decrease in CDAI score from baseline), a greater proportion of patients in groups treated with ENTYVIO as compared to placebo discontinued corticosteroids by Week 52 and were in clinical remission at Week 52 (Table 8).
Table 8. Proportion of Patients Meeting Efficacy Endpoints at Week 52* (CD Trial III)
|
|
Placebo† N=153 |
ENTYVIO Every 8 Weeks N=154 |
p-value | Treatment Difference and 95% CI |
|---|---|---|---|---|
| Clinical remission‡ at Week 52 | 22% | 39% | 0.001 | 17% (7%, 28%) |
| Clinical response§ at Week 52 | 30% | 44% | 0.013 | 13% (3%, 24%) |
| Corticosteroid-free clinical remission# | 16%# | 32%# | 0.015 | 16% (3%, 29%) |
*This group includes patients that were not in clinical remission at Week 6. Patients must have achieved clinical response (defined as ≥70 decrease in CDAI from baseline) at Week
6 to continue into CD Trial III.
†The placebo group includes those patients who received ENTYVIO at Week 0 and Week 2, and were randomized to receive placebo from Week 6 through Week 52
‡Clinical remission: CDAI ≤150
§Clinical response: ≥100 decrease in CDAI from baseline
#Corticosteroid-free clinical remission: Assessed in the subgroup of patients who were receiving corticosteroids at baseline and who were in clinical response (defined as ≥70 decrease in CDAI from baseline) at Week 6 (n=82 for placebo and n=82 for ENTYVIO every eight weeks). Corticosteroid-free clinical remission was defined as the proportion of patients in this subgroup that discontinued corticosteroids by Week 52 and were in clinical remission at Week 52.
Source: Drug Label, Table 6
In CD Trial III a greater percentage of patients in groups treated with ENTYVIO as compared to placebo were in clinical remission (defined as CDAI score ≤150) at Week 52. A greater percentage of patients in groups treated with ENTYVIO as compared to placebo had a clinical response (defined as ≥100 decrease in CDAI score from baseline) at Week 52. In the subgroup of patients who were receiving corticosteroids at baseline and who were in clinical response at Week 6 (defined as ≥70 decrease in CDAI score from baseline), a greater proportion of patients in groups treated with ENTYVIO as compared to placebo discontinued corticosteroids by Week 52 and were in clinical remission at Week 52)
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
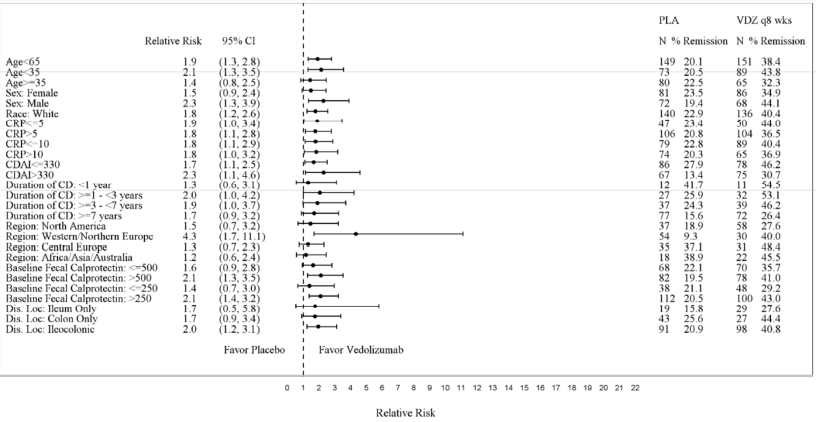
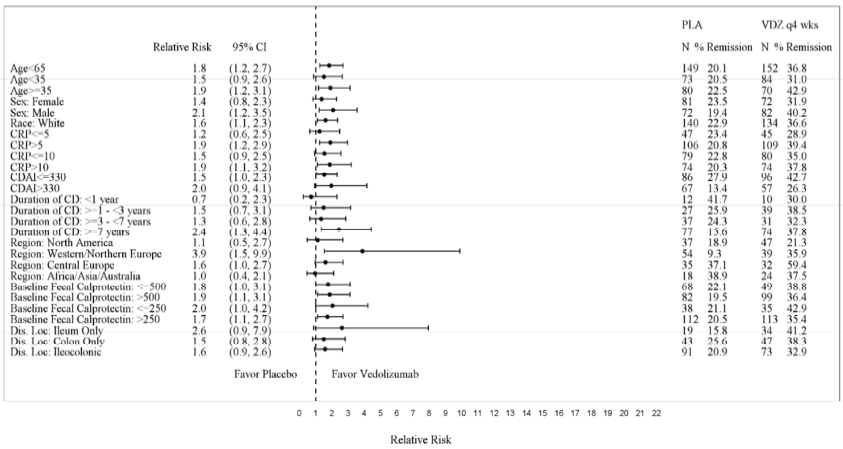
Subgroup analyses were conducted for sex, race and age. Figures 5, 6, 7, and 8 show the demographic subgroup analyses for CD Trial I, CD Trial II, CD Trial III (Q8W vs. Placebo), and CD Trial III (Q4W vs. Placebo).
- Sex: Subgroup analyses were conducted. Response to ENTYVIO was consistent between men and women.
- Race: Subgroup analyses were conducted, but the number of patients in the non-white subgroups was limited. Therefore, differences in response to ENTYVIO could not be determined.
- Age: Subgroup analyses were conducted, but the number of patients above 65 years of age was limited; therefore, differences between those above and below 65 years of age could not be determined. Response to ENTYVIO was consistent in patients above and below 35 years of age.
Figure 5: CD Trial I Efficacy Demographic Subgroup Analyses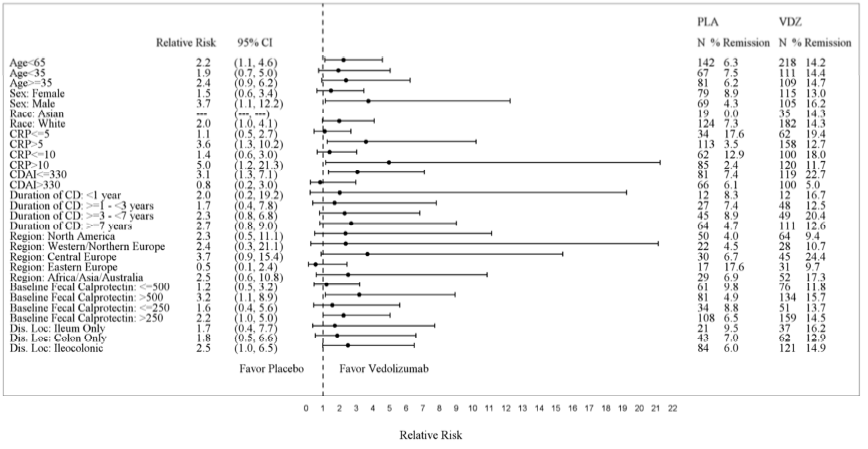
| Placebo | VDZ | |||||
|---|---|---|---|---|---|---|
| Relative Risk | (95% CI) | N | % | N | % | |
| Age<> | 2.2 | (1.1, 4.6) | 142 | 6.3 | 218 | 14.2 |
| Age<> | 1.9 | (0.7, 5.0) | 67 | 7.5 | 111 | 14.4 |
| Age≥35 | 2.4 | (0.9, 6.2) | 81 | 6.2 | 109 | 14.7 |
| Female | 1.5 | (0.6, 3.4) | 79 | 8.9 | 115 | 13 |
| Male | 3.7 | (1.1, 12.2) | 69 | 4.3 | 105 | 16.2 |
| Asian | *** | ( ***, ***) | 19 | 0 | 35 | 14.3 |
| White | 2 | (1.0, 4.1) | 124 | 7.3 | 182 | 14.3 |
Source: FDA Medical Review, p 65
Figure 6: CD Trial II Efficacy Demographic Subgroup Analyses
| Placebo | VDZ | |||||
|---|---|---|---|---|---|---|
| Relative Risk | (95% CI) | N | % Remission | N | % Remission | |
| Age<> | 1.6 | (1.0, 2.5) | 202 | 11.9 | 206 | 18.4 |
| Age<> | 2.4 | (1.1, 5.0) | 105 | 8.6 | 88 | 20.5 |
| Age≥35 | 1.2 | (0.6, 2.1) | 102 | 15.7 | 121 | 18.2 |
| Female | 1.1 | (0.6, 2.0) | 118 | 15.3 | 118 | 16.9 |
| Male | 2.8 | (1.2, 6.3) | 89 | 7.9 | 91 | 22 |
| White | 1.6 | (1.0, 2.6) | 186 | 11.8 | 188 | 19.1 |
Source: FDA Medical Review, p 65
Figure 7: CD Trial III Q8W vs. Placebo Efficacy Demographic Subgroup Analyses
| Placebo | VDZ | |||||
|---|---|---|---|---|---|---|
| Relative Risk | (95% CI) | N | % Remission | N | % Remission | |
| Age<> | 1.9 | (1.3, 2.8) | 149 | 20.1 | 151 | 38.4 |
| Age<> | 2.1 | (1.3, 3.5) | 73 | 20.5 | 89 | 43.8 |
| Age≥35 | 1.4 | (0.8, 2.5) | 80 | 22.5 | 65 | 32.3 |
| Female | 1.5 | (0.9, 2.4) | 81 | 23.5 | 86 | 34.9 |
| Male | 2.3 | (1.3, 3.9) | 72 | 19.4 | 68 | 44.1 |
| White | 1.8 | (1.2, 2.6) | 140 | 22.9 | 136 | 40.4 |
Source: FDA Medical Review, p 83
Figure 8: CD Trial III Q4W vs. Placebo Efficacy Demographic Subgroup Analyses
| Placebo | VDZ | |||||
|---|---|---|---|---|---|---|
| Relative Risk | (95% CI) | N | % Remission | N | % Remission | |
| Age<> | 1.8 | (1.2, 2.7) | 149 | 20.1 | 152 | 36.8 |
| Age<> | 1.5 | (0.9, 2.6) | 73 | 20.5 | 84 | 31 |
| Age≥35 | 1.9 | (1.2, 3.1) | 80 | 22.5 | 70 | 42.9 |
| Female | 1.4 | (0.8, 2.3) | 81 | 23.5 | 72 | 31.9 |
| Male | 2.1 | (1.2, 3.5) | 72 | 19.4 | 82 | 40.2 |
| White | 1.6 | (1.1, 2.3) | 140 | 22.9 | 134 | 36.6 |
Source: FDA Medical Review, p 84
What are the results of the safety study?
Adverse reactions were reported in 52% of patients treated with ENTYVIO and 45% of patients treated with placebo (UC Trials I and II: 49% with ENTYVIO and 37% with placebo; CD Trials I and III: 55% with ENTYVIO and 47% with placebo). Serious adverse reactions were reported in 7% of patients treated with ENTYVIO compared to 4% of patients treated with placebo (UC Trials I and II: 8% with ENTYVIO and 7% with placebo; CD Trials I and III: 12% with ENTYVIO and 9% with placebo).
The most common adverse reactions (reported by ≥3% of patients treated with ENTYVIO in the UC Trials I and II and CD Trials I and III combined group and ≥1% higher than in combined placebo group) were nasopharyngitis, headache, arthralgia, nausea, pyrexia, upper respiratory tract infection, fatigue, cough, bronchitis, influenza, back pain, rash, pruritus, sinusitis, oropharyngeal pain and pain in extremities (see Table 9).
Table 9. Adverse Reactions in ≥3% of ENTYVIO-treated Patients and ≥1%
Higher than in Placebo (UC Trials I and II* and CD Trials I and III*)
| Adverse Reaction | ENTYVIO† (N=1434) |
Placebo‡ (N=297) |
|---|---|---|
| Nasopharyngitis | 13% | 7% |
| Headache | 12% | 11% |
| Arthralgia | 12% | 10% |
| Nausea | 9% | 8% |
| Pyrexia | 9% | 7% |
| Upper respiratory tract infection | 7% | 6% |
| Fatigue | 6% | 3% |
| Cough | 5% | 3% |
| Bronchitis | 4% | 3% |
| Influenza | 4% | 2% |
| Back pain | 4% | 3% |
| Rash | 3% | 2% |
| Pruritus | 3% | 1% |
| Sinusitis | 3% | 1% |
| Oropharyngeal pain | 3% | 1% |
| Pain in extremities | 3% | 1% |
*Data from patients receiving open-label ENTYVIO treatment at Weeks 0 and 2 (prior to entry into UC Trial II and CD Trial III) and from Weeks 6 to 52 (non-responders at
Week 6 of UC Trial I and CD Trial I) are included.
†Patients who received ENTYVIO for up to 52 weeks.
‡Patients who received placebo for up to 52 weeks.
Source: Drug Label, Table 1