Drug Trials Snapshot: Entyvio (vedolizumab) to Treat Crohn's Disease
Disclaimer: The Drug Trials Snapshot provides information about who was in the clinical trials that led to the FDA approval of this drug. This website shows who participated in these studies by sex, race, and age groups. You can get more information about this drug from the ENTYVIO Drug Label and your doctor or health care professional.
ENTYVIO (vedolizumab)
To Treat Crohn’s Disease
(en ti’ vee oh)
Takeda Pharmaceuticals U.S.A., Inc.
Approval date: May 20, 2014
DRUG TRIALS SUMMARY:
What is the drug for?
ENTYVIO is used to treat adult patients with moderate to severe Crohn‘s disease (CD) when certain other Crohn's disease medications have not worked well enough or cannot be tolerated.
How does one use this drug?
ENTYVIO is administered by intravenous (IV) infusion through a vein, over approximately a 30 minute period. After the first dose, it is given again at two and six weeks, then every eight weeks thereafter.
What are the benefits?
ENTYVIO was better than the placebo at helping to get CD under control (achieve remission). It may help reduce or stop the need to take corticosteroid medicines.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: Subgroup analyses were conducted. Response to ENTYVIO was consistent between men and women.
- Race: Subgroup analyses were conducted, but the number of patients in the non-white subgroups was limited. Therefore, differences in response to ENTYVIO could not be determined.
- Age: Subgroup analyses were conducted. The number of patients above 65 years of age was limited; therefore, differences in response between patients above and below 65 years of age could not be determined. Response to ENTYVIO was consistent in patients above and below 35 years of age.
What are the possible side effects?
The most common side effects that occurred in patients treated with ENTYVIO in clinical trials included common cold, headache, joint pain, nausea, fever, infections of the nose and throat, tiredness, cough, bronchitis, flu, back pain, rash, itching, sinus infection, throat pain, and pain in extremities. Other risks associated with ENTYVIO can include infusion and serious allergic reactions, serious infections, and liver injury. Although not reported with ENTYVIO, it may be possible for a person to get progressive multifocal leukoencephalopathy (PML) (a rare, serious brain infection caused by a virus); PML can result in death or severe disability, and there is no known treatment, prevention, or cure for PML.
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age in a combined group of patients with Crohn’s disease or Ulcerative Colitis.
- Sex: The frequency of side effects was similar between men and women.
- Race: The number of patients in the non-white subgroups was limited. Therefore, differences among races could not be detected.
- Age: The number of patients above 65 years of age was limited. Therefore, differences between those above and below 65 years of age could not be detected.
WHO WAS IN THE STUDY?
Who participated in the study?
The FDA approved ENTYVIO based on evidence three clinical trials in patients with moderate to severe Crohn’s Disease. The trials were conducted in North America, Europe, Asia, Africa and Australia.
Figure 1 summarizes how many men and women were enrolled in the clinical trials used to evaluate efficacy.
Figure 1. Baseline Demographics by Sex
Source: Adapted from Statistical Review, Table 1, 12, and 24
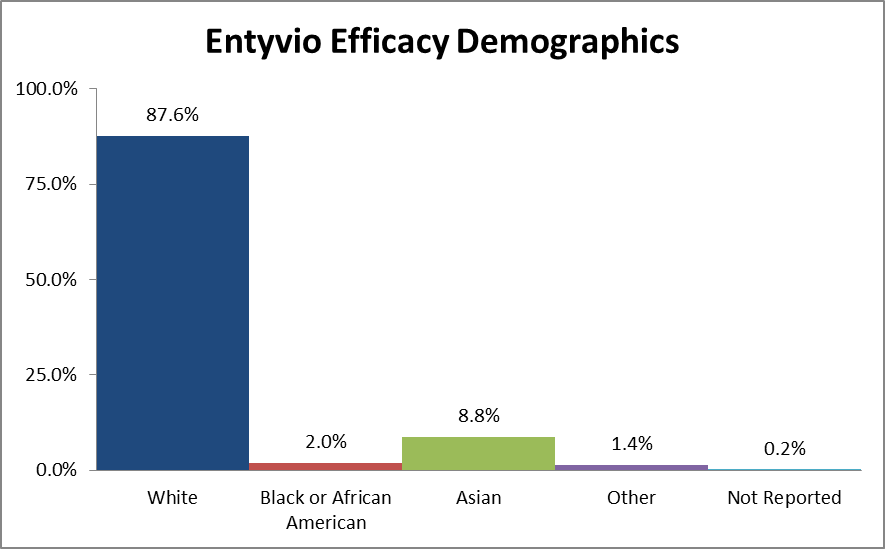
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trials used to evaluate efficacy.
Source: Adapted from Statistical Review, Table 1, 12, and 24
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 1090 | 87.6% |
| Black or African American | 25 | 2.0% |
| Asian | 110 | 8.8% |
| Other | 18 | 1.4% |
| Not Reported | 2 | 0.2% |
Source: Adapted from Statistical Review, Table 1, 12, and 24
Figure 3 summarizes how many men and women were enrolled in the clinical trials used to assess safety.
Figure 3. Demographics of Safety Trials by Sex
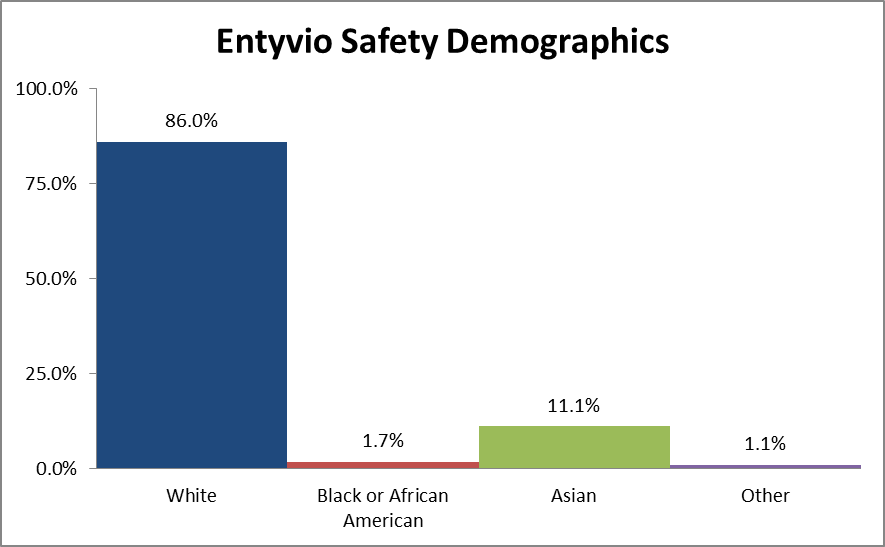
Figure 4 summarizes the percentage of patients by race enrolled in the clinical trials used to assess safety.
Figure 4. Demographics of Safety Trials by Race
Source: Adapted from FDA Medical Review, Table 10 and 12
Table 2. Demographics of Safety Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 1729 | 86.0% |
| Black or African American | 35 | 1.7% |
| Asian | 224 | 11.1% |
| Other | 22 | 1.1% |
Source: Adapted from FDA Medical Review, Table 10 and 12
How was the study designed?
The FDA approval of ENTYVIO for Crohn’s Disease was based on three randomized, double-blind, placebo-controlled trials in adult patients with moderately to severely active Crohn’s Disease. That means neither the patients nor the health care professionals administering the drug knew if the patient was taking the actual drug or a placebo until the study was complete.
What are the results of the efficacy study?
Efficacy assessments were at 6 weeks and 52 weeks. Results showed that a greater percentage of participants treated with ENTYVIO compared to placebo achieved clinical response, achieved clinical remission, and achieved corticosteroid-free clinical remission.
What are the results of the safety study?
The most common side effects in patients treated with ENTYVIO include common cold, headache, joint pain, nausea, fever, infections of the nose and throat, tiredness, cough, bronchitis, flu, back pain, rash, itching, sinus infection, throat pain, and pain in extremities. ENTYVIO can increase the risk of infusion and serious allergic reactions, serious infections, and liver injury. Although not reported with ENTYVIO, it may be possible for a person to get progressive multifocal leukoencephalopathy (PML) (a rare, serious brain infection caused by a virus); PML can result in death or severe disability, and there is no known treatment, prevention, or cure for PML.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
MEDICAL REVIEW (PDF - 28MB)
DRUG LABEL (PDF - 405KB)