MAVYRET Drug Trials Snapshot
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the MAVYRET Prescribing Information for complete information.
MAVYRET (glecaprevir and pibrentasvir)
MAV-ih-reht
AbbVie Inc.
Approval date: August 3, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MAVYRET is a drug for the treatment of adults who have a specific type of Hepatitis C virus (HCV) infection, called chronic Hepatitis C virus genotypes 1, 2, 3, 4, 5 or 6 infection. Hepatitis C is a viral disease that causes inflammation of the liver that can lead to decreased liver function, cirrhosis, liver failure, liver cancer or death.
MAVYRET is a combination of two anti-viral drugs: glecaprevir and pibrentasvir. It is intended to be used in patients who do not have cirrhosis or who have early stage cirrhosis.
How is this drug used?
MAVYRET is a tablet. Three tablets are taken once a day. Treatment duration is different depending on viral genotype, prior treatment history and cirrhosis status.
What are the benefits of this drug?
MAVYRET may clear the body of the hepatitis C virus as measured by a blood test after finishing treatment.
What are the benefits of this drug?
The tables below summarize efficacy results for the clinical trials in patients with HCV infection. The primary endpoint was Sustained Virologic Response (SVR) measured at 12 weeks after cessation of treatment and was defined as HCV RNA below the lower limit of quantification.
Table 2. Efficacy in Treatment-Naïve and PRS Treatment-Experienced With HCV Genotype 1 Infection and Without Cirrhosis (ENDURANCE-1 Trial)
| Genotype 1 MAVYRET 8 Weeks (N=351) | ||
|---|---|---|
| SVR12 | 99% (348/351) | |
| Outcome for Subjects Without SVR12 | ||
| On-treatment VF | 1%> | |
| Relapse | 0/349 | |
| Other* | 1%> | |
| PRS= combinations of peginterferon, ribavirin and/or sofosbuvir VF= virologic failure * Includes subjects who discontinued due to adverse event, lost to follow-up, or subject withdrawal. | ||
MAVYRET Prescribing Information
Table 3. Efficacy in Treatment-Naïve and PRS Treatment-Experienced Adults With HCV Genotypes 2, 4, 5 or 6 Infection Without Cirrhosis*
| SURVEYOR-2 MAVYRET 8 Weeks | ENDURANCE-4 and SURVEYOR-1 MAVYRET 12 Weeks | |||||
|---|---|---|---|---|---|---|
| GT2 N=197 | GT4 N=46 | GT5 N=2 | GT6 N=10 | GT5 N=27 | GT6 N=30 | |
| SVR 12 | 98% (193/197) | 93% (43/46) | 100% (2/2) | 100% (10/10) | 100% (27/27) | 100% (30/30) |
| Outcome for Subjects Without SVR12 | ||||||
| On Treatment VF | 0/197 | 0/46 | 0/2 | 0/10 | 0/27 | 0/30 |
| Relapse | 1% (2/195) | 0/45 | 0/2 | 0/10 | 0/26 | 0/29 |
| Other** | 1% (2/197) | 7% (3/46) | 0/2 | 0/10 | 0/27 | 0/30 |
| *Pooled open-label trials: SURVEYOR-2 (Part 2 and Part 4), ENDURANCE-4 and SURVEYOR-1 (Part 2): GT=genotype; VF= virologic failure PRS= combinations of peginterferon, ribavirin and/or sofosbuvir ** Includes subjects who discontinued due to adverse event, lost to follow-up, or subject withdrawal. | ||||||
MAVYRET Prescribing Information
Table 4. Efficacy in Treatment-Naïve and PRS Treatment-Experienced Adults With HCV Genotype 1, 2, 4, 5 or 6 Infection With Compensated Cirrhosis (EXPEDITION-1 Trial)
| SVR12 | MAVYRET 12 Weeks (N=146) | |||||
|---|---|---|---|---|---|---|
| Total (all GTs) (N=146) | GT1 (N=90) | GT2 (N=31) | GT4 (N=16) | GT5 (N=2) | GT6 (N=7) | |
| 99% (145/146) | 99% (89/90) | 100% (31/31) | 100% (16/16) | 100% (2/2) | 100% (7/7) | |
| Outcome for Subjects Without SVR12 | ||||||
| On-treatment VF | 0/146 | 0/90 | 0/31 | 0/16 | 0/2 | 0/7 |
| Relapse | 1%> (1/144) | 1% (1/88) | 0/31 | 0/16 | 0/2 | 0/7 |
| PRS= combinations of peginterferon, ribavirin and/or sofosbuvir GT = genotype; VF = virologic failure | ||||||
MAVYRET Prescribing Information
Table 5. Efficacy in Treatment-Naïve, HCV Genotype 3-Infected Subjects Without Cirrhosis (ENDURANCE-3 Trial)
| MAVYRET1 8 Weeks (N=157) | MAVYRET 12 Weeks* (N=233) | DCV+SOF 12 Weeks (N=115) | |
|---|---|---|---|
| SVR12 | 94.9% (149/157) | 95.3% (222/233)* | 96.5% (111/115) |
| Outcome for Subjects Without SVR12 | |||
| On-treatment VF | 1% (1/157) | 1%> | 0/115 |
| Relapse | 3% (5/150) | 1% (3/222) | 1% (1/114) |
| Other2 | 1% (2/157) | 3% (7/233) | 3% (3/115) |
| DVC+SOF= combination of daclatasvir and sofosbuvir ; VF=virologic failure 1MAVYRET 8 weeks was a non-randomized treatment arm. 2 Includes subjects who discontinued due to adverse event, lost to follow-up, or subject withdrawal. * Data for MAVYRET 12-week treatment is displayed to reflect the original randomized study design. The treatment difference (95% confidence interval) was -1.2% (-5.6, 3.1) between the randomized arms of MAVYRET 12 weeks and DCV + SOF 12 weeks. | |||
MAVYRET Prescribing Information
Table 6. Efficacy in Treatment-Naïve or PRS Treatment-Experienced, HCV Genotype 3-Infected Adults Without Cirrhosis or With Compensated Cirrhosis (SURVEYOR-2 Trial Part 3)
| Treatment-Naïve With Compensated Cirrhosis | PRS Treatment-Experienced Without Cirrhosis or With Compensated Cirrhosis | |
|---|---|---|
| MAVYRET 12 Weeks (N=40) | MAVYRET 16 Weeks (N=69) | |
| SVR12 | 98% (39/40) | 96% (66/69) |
| Outcome for Subjects Without SVR12 | ||
| On-treatment VF | 0/40 | 1% (1/69) |
| Relapse | 0/39 | 3% (2/68) |
| Other* | 3% (1/40) | 0/69 |
| SVR12 by Cirrhosis Status | ||
| Without Cirrhosis | NA | 95% (21/22) |
| With Compensated Cirrhosis | 98% (39/40) | 96% (45/47) |
| PRS= combinations of peginterferon, ribavirin and/or sofosbuvir VF=virologic failure * Includes subjects who discontinued due to adverse event, lost to follow-up, or subject withdrawal. | ||
MAVYRET Prescribing Information
Table 7. Efficacy in HCV Genotype 1-Infected Adults Who Are NS3/4A PI-Experienced or NS5A Inhibitor-Experienced, Without Cirrhosis or With Compensated Cirrhosis (MAGELLAN-1 Trial)
| PI-Experienced1 (NS5A Inhibitor- naïve) | NS5A Inhibitor- Experienced2 (PI-naïve) | |
|---|---|---|
| MAVYRET 12 Weeks (N=25) | MAVYRET 16 Weeks (N=17) | |
| SVR12 | 92% (23/25) | 94% (16/17) |
| Outcome for Subjects Without SVR | ||
| On-treatment Virologic Failure | 0/25 | 6% (1/17) |
| Relapse | 0/25 | 0/16 |
| Other3 | 8% (2/25) | 0/17 |
| PI= protease inhibitor 1 Includes patients who were treated with a regimen containing an NS3/4A PI (simeprevir with sofosbuvir, or simeprevir, boceprevir, or telaprevir with pegylated interferon and ribavirin) and without prior treatment with an NS5A inhibitor. 2 Includes patients who were treated with a regimen containing an NS5A inhibitor (ledipasvir with sofosbuvir or daclatasvir with pegylated interferon and ribavirin) and without prior treatment with an NS3/4A PI. 3 Includes patients who discontinued due to adverse event, lost to follow-up, or patients withdrawal. | ||
MAVYRET Prescribing Information
Treatment-Naïve and PRS Treatment-Experienced Adults With Chronic Kidney Disease Stage 4 and 5 and Chronic HCV Infection Without Cirrhosis or With Compensated Cirrhosis (EXPEDITION-4 Trial) In this open-label, single-arm trial, patients with moderate to severe kidney disease, including patients who required dialysis were studied. The overall SVR12 rate was 98% and no patients experienced virologic failure. |
MAVYRET Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: MAVYRET worked similarly in men and women.
- Race: MAVYRET worked similarly in patients of White, Asian and Black or African American race.
- Age: MAVYRET worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The Table below presents an example of some of the efficacy results by subgroup in pooled sample of patients treated with MAVYRET for 12 weeks. Results were similar in patients treated with MAVYRET for 8 and 16 weeks. However, for some subgroups the numbers are too small to make meaningful comparisons.
Table 8. Pooled SVR12 by Age, Race, sex, and Region in Treatment-Naïve Patients
| MAVYRET 12 weeks treatment | GT1 | GT2 | GT3 | GT4 | GT5 | GT6 |
|---|---|---|---|---|---|---|
| Pooled Trials* | 590, 172 | 172, 868, 464 | 594, 868 | 583, 172, 867,868 | 583, 172,867, 868 | 583, 172,867, 868 |
| N total | 283 | 187 | 297 | 75 | 23 | 33 |
| Age | ||||||
| 65=""> | 248/249(99.6) | 129/131(98.5) | 273/285(95.8) | 67/67(100) | 15/15(100) | 30/30(100) |
| ≥ 65 years | 34/34(100) | 56/56(100) | 12/12(100) | 8/8(100) | 8/8(100) | 3/3(100) |
| Race | ||||||
| White | 251/251(100) | 127/129(98.4) | 256/265(96.6) | 61/61(100) | 16/16(100) | 4/4(100) |
| Black | 14/15(93.3) | 7/7(100) | 4/4(100) | 10/10(100) | 3/3(100) | 0 |
| Asian | 15/15(100) | 47/47(100) | 18/20(90) | 4/4(100) | 1/1(100) | 28/28(100) |
| Other | 2/2(100) | 4/4(100) | 4/5(80) | 0 | 2/2(100) | 1/1(100) |
| Sex | ||||||
| Men | 140/140(100) | 88/90(97.8) | 148/158(93.7) | 46/46(100) | 13/13(100) | 15/15(100) |
| Women | 142/143(99.3) | 97/97(100) | 137/139(98.6) | 29/29(100) | 10/10(100) | 18/18(100) |
| Region | ||||||
| North America | 103/104(99.0) | 66/68(97.1) | 122/125(97.6) | 22/22(100) | 2/2(100) | 16/16(100) |
| Europe | 141/141(100) | 72/72(100) | 85/93(91.4) | 50/50(100) | 13/13(100) | 10/10(100) |
| Rest of the World | 38/38(100) | 47/47(100) | 78/79(96) | 3/3(100) | 8/8(100) | 7/7(100) |
*Trials include: 590 (ENDURANCE-1), 172 (EXPEDITION-1), 867 (SURVEYOR-1), 868 (SURVEYOR-2), 464 (ENDURANCE 2), 583 (ENDURANCE-4), and 594 (ENDURANCE-3)
FDA Statistical review
What are the possible side effects?
MAVYRET may cause serious liver problems including liver failure and death in patients who had hepatitis B virus infection. This is because the hepatitis B virus could become active again (called reactivation) during or after treatment of hepatitis C virus with MAVYRET.
The most common side effects of MAVYRET are headache and tiredness.
What are the possible side effects (results of trials used to assess safety)?
Below is the summary of the most common adverse reactions with frequency of 5% or greater that were observed in controlled trials.
Table 9. Adverse Reactions Reported in ≥5% of Treatment-Naïve and PRS-Experienced Adults without Cirrhosis Receiving MAVYRET for 12 Weeks (ENDURANCE-2 Trial)
Adverse Reaction | MAVYRET | Placebo |
|---|---|---|
Headache | 9 | 6 |
Nausea | 6 | 2 |
Diarrhea | 5 | 2 |
MAVYRET Prescribing Information
Table 10. Adverse Reactions Reported in ≥5% of Treatment-Naïve Adults without Cirrhosis Receiving MAVYRET for 8 Weeks or 12 Weeks in (ENDURANCE-3 Trial)
Adverse Reaction | MAVYRET* | MAVYRET | daclatasvir +sofosbuvir |
|---|---|---|---|
Headache | 16 | 17 | 15 |
Fatigue | 11 | 14 | 12 |
Nausea | 9 | 12 | 12 |
Diarrhea | 7 | 3 | 3 |
* The 8 week arm was a non-randomized treatment arm.
MAVYRET Prescribing Information
Overall, in nine trials of patients infected with HCV genotype 1, 2, 3, 4, 5, or 6 who received MAVYRET for 8, 12 or 16 weeks, the most common adverse reactions, all grades, observed in greater than or equal to 5% of patients were headache (13%), fatigue (11%), and nausea (8%).
Were there any differences in side effects among sex, race and age?
- Sex: The risk of side effects was similar in men and women.
- Race: The risk of side effects was similar in patents of White, Asian and Black or African American race.
- Age: The risk of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize the frequency of all adverse events (regardless if the adverse events were considered related to MAVYRET) for the safety population by subgroup.
Table 11. Subgroup Analyses of Adverse Events by Sex
| Men | Women |
|---|---|---|
Any Adverse Event | 853 (64.7) | 750 (71.4) |
Any Adverse Event Grade ≥3 | 54 (4.1) | 36 (3.4) |
Any Serious Adverse Event | 46 (3.5) | 27 (2.6) |
Table 12. Subgroup Analyses of Adverse Events by Race
| White | Black or African American* | Asian |
|---|---|---|---|
Any Adverse Event | 1317 (69.4) | 104 (67.5) | 155 (57) |
Any Adverse Event Grade ≥3 | 71 (3.7) | 11 (7.1) | 5 (1.8) |
Any Serious Adverse Event | 55 (2.9) | 11 (7.1) | 5 (1.8) |
*The EXPEDITION-4 trial conducted in patients with CKD, patients who are sicker at baseline and would be expected to have a higher number of adverse events, enrolled a larger percentage of Black or African American (25/104) than other trials.
Table 13. Subgroup Analyses of Adverse Events by Age Group
| 65=""> | ≥65 years |
|---|---|---|
Any Adverse Event | 1,392 (68.2) | 211 (64.3) |
Any Adverse Event Grade ≥3 | 66 (3.2) | 24 (7.3) |
Any Serious Adverse Event | 49 (2.4) | 24 (7.3) |
Adapted from FDA clinical review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved MAVYRET based on evidence from nine clinical trials of 2369 patients with chronic hepatitis C virus infection. In these trials some patients were previously treated for hepatitis C and some were never treated before. Some patients had cirrhosis and some did not. The trials were conducted in the United States, Canada, Europe, Australia, and New Zealand.
The figure below summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical trial report
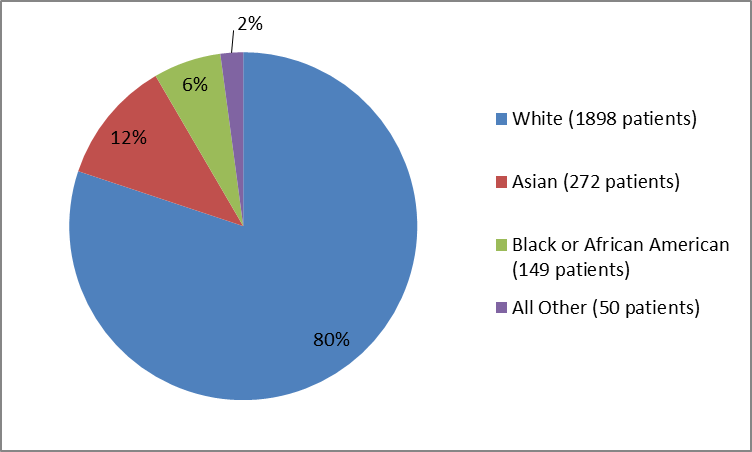
Figure 2 and Table 1 summarize the percentage of patients in the clinical trials.
Figure 2. Baseline Demographics by Race
Clinical trial report
Table 1. Demographics of Trials by Race
Race | Number of Patients | Percentage |
|---|---|---|
White | 1898 | 80 |
Asian | 272 | 12 |
Black or African American | 149 | 6 |
Native Hawaiian or Pacific Islander | 17 | 1 |
Multiple | 17 | 1 |
American Indian or Alaska Native | 13 | 1 |
Not reported | 3 | less than 1 |
Clinical trial report
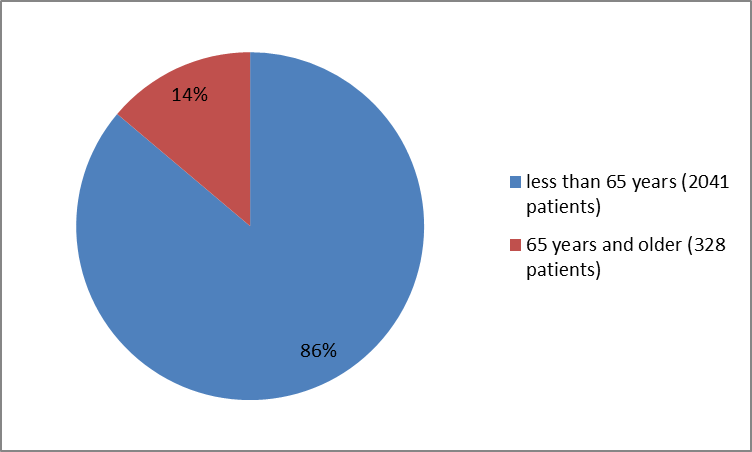
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical trial report
Who participated in the trials?
The table below summarizes demographics of patients enrolled in the nine clinical trials.
Table 14. Patient Demographics
| Demographic Parameters | All Trials combined(N=2369) |
|---|---|
| Sex | |
| Men | 1318 (55.6%) |
| Women | 1051 (44.4%) |
| Age | |
| Mean years (SD) | 52.5 (11.8) |
| Median (years) | 54 |
| Min, max (years) | 19, 88 |
| AgeGroup | |
| 65=""> | 2041 (86.2%) |
| ≥ 65 years | 328 (13.8%) |
| Race | |
| White | 1898 (80.1%) |
| Asian | 272 (11.5%) |
| Black or African American | 149 (6.3%) |
| Native Hawaiian or Pacific Islander | 17 (0.7) |
| Multiple | 17 (0.7) |
| American Indian or Alaska Native | 13 (0.5) |
| Not disclosed | 3 (0.1%) |
| Ethnicity | |
| Hispanic or Latino | 211 (8.9%) |
| Not Hispanic or Latino | 1522 (64.2%) |
| Not disclosed | 636 (26.8%) |
| Region | |
| United States | 750 (31.7%) |
| Non-US | |
| Canada | 178 (7.5%) |
| Australia | 176 (7.4%) |
| New Zealand | 110 (4.6%) |
| Europe | 891 (37.6%) |
Adapted from FDA review
How were the trials designed?
FDA approved MAVYRET based on evidence from nine clinical trials of 2369 patients with chronic hepatitis C virus infection genotypes 1-6.
Across the trials some patients were previously treated for hepatitis C and some were never treated before. Some patients had cirrhosis and some did not. One trial included patients who also had HIV infection and one trial included patients who had advanced kidney disease, including some patients who required dialysis.
Each trial was designed differently. Patients received MAVYRET for 8, 12 or 16 weeks Seven trials included treatment with MAVYRET only, and two trials compared MAVYRET treatment to either placebo or an approved daclatasvir + sofosbuvir HCV regimen.
All trials measured the blood level of hepatitis virus C before, during, and after treatment.
How were the trials designed?
The efficacy and safety of MAVYRET were established in nine clinical trials with a total of 2369 adult patients who had chronic hepatitis C genotypes 1-6 infection. Across the trials, different populations were studied: treatment naïve and treatment experienced, with no cirrhosis or with compensated cirrhosis, some with HIV co-infection and some with severe renal impairment (CKD Stages 4 and 5).
Two trials were controlled trials: one was randomized, double-blind, placebo-controlled, and the other was randomized, open-label active-controlled (daclatasvir + sofosbuvir). All other trials were non-controlled, open label either one-or multi arm trials.
In all trials, the primary efficacy outcome was sustained virologic response (SVR12) defined as HCV RNA less than lower limit of quantification 12 weeks after the end of treatment which lasted 8, 12 or 16 weeks.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.