Drugs Trials Snapshot: Danyelza
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the DANYELZA Prescribing Information for complete information.
DANYELZA (naxitamab-gqgk)
(dan-YEL-zah)
Y-mAbs Therapeutics, Inc.
Approval date: November 25, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DANYELZA is a drug used in combination with a medicine called granulocyte-macrophage colony-stimulating factor (GM-CSF) to treat children 1 year of age and older and adults with high-risk neuroblastoma in bone or bone marrow whose tumor
- did not respond to or has come back after previous treatments and
- has shown a partial response, minor response, or stable disease to prior therapy.
Neuroblastoma is a rare cancer that develops from nerve cells anywhere in the body and most often occurs in young children.
How is this drug used?
DANYELZA is an injection given by a healthcare professional directly into a vein (intravenous infusion) on Days 1, 3 and 5 of each treatment cycle. GM-CSF is given under the skin (subcutaneously) daily starting 5 days prior to DANYELZA and continuing until the last day of DANYELZA administration of each cycle.
What are the benefits of this drug?
In one trial, approximately 45 percent of patients receiving DANYELZA with GM-CSF experienced complete or partial shrinkage of their cancer; for 30% of those patients, shrinkage lasted 6 months or longer.. In another trial, 34% patients receiving DANYELZA with GM-CSF experienced complete or partial shrinkage of their cancer; for 23% of those patients, shrinkage lasted 6 months or longer for.
DANYELZA was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for individual trials based on overall response rate (ORR) as determined by independent pathology and imaging review according to the revised International Neuroblastoma Response Criteria (INRC) and duration of response (DOR), as determined by an independent pathology and imaging review supplemented by long-term follow-up data.
Table 1. Efficacy Results for the Clinical Trial 1
|
DANYELZA with GM-CSF |
|
|---|---|
|
Overall response ratea (95% CI) |
45% (24%, 68%) |
|
Complete response rate |
36% |
|
Partial response rate |
9% |
|
Duration of response |
|
|
Median (95% CI), months |
6.2 (4.9, NE) |
|
Patients with DOR ≥ 6 months |
30% |
CI = confidence interval
NE: not estimable.
aOverall response rate is defined as a complete or partial response according to the revised INRC (2017) that was confirmed by at least one subsequent assessment. Responses were observed in the bone, bone marrow, or both bone and bone marrow.
Table 2. Efficacy Results for the Clinical Trial 2
|
DANYELZA with GM-CSF |
|
|---|---|
|
Overall response ratea (95% CI) |
34% (20%, 51%) |
|
Complete response rate |
26% |
|
Partial response rate |
8% |
|
Duration of Response |
|
|
Patients with DOR ≥ 6 months |
23% |
CI = confidence interval,
aOverall response rate is defined as a complete or partial response according to the revised INRC (2017) that was confirmed by at least one subsequent assessment. Responses were observed in the bone, bone marrow, or both bone and bone marrow.
DANYELZA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: DANYELZA was similarly effective in boys and girls.
- Race: The majority of patients were White; therefore, differences in response to DANYELZA among different race groups could not be determined.
- Age: The majority of patients were 12 years or younger, therefore, differences in response to DANYELZA among patients of different age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The following tables summarize efficacy results by demographic subgroups in individual trials. Given the small sample size for the subgroups, results must be interpreted with caution.
Table 3. Subgroup Analysis of Trial 1
|
N |
ORR (95% CI) |
|
|---|---|---|
|
Sex |
||
|
Female |
13 |
54% (25, 81) |
|
Male |
9 |
33% (7, 70) |
|
Race |
||
|
White |
10 |
70% (35, 93) |
|
Asian |
11 |
27% (6, 61) |
|
Other |
1 |
0% (0, 98) |
|
Age |
||
|
< 6 years |
12 |
42% (15, 72) |
|
6 – 12 years |
10 |
50% (19, 81) |
|
≥ 12 years |
0 |
- |
Table 4. Subgroup Analysis of Trial 2
|
|
N |
ORR (95% CI) |
|---|---|---|
|
Sex |
||
|
Female |
19 |
37% (16, 62) |
|
Male |
19 |
32% (13, 57) |
|
Race |
||
|
White |
28 |
25% (11, 45) |
|
Asian |
3 |
67% (9, 99) |
|
Other |
7 |
57% (18, 90) |
|
Age |
||
|
< 6 years |
20 |
40% (19, 64) |
|
6 – 12 years |
13 |
38% (14, 68) |
|
≥ 12 years |
5 |
0% (0, 52) |
FDA Review
What are the possible side effects?
DANYELZA may cause serious side effects including
- life-threatening reactions related to the infusion,
- severe pain and nerve damage,
- inflammation of the spinal cord,
- brain damage called Reversible Posterior Leukoencephalopathy,
- increased blood pressure,
- harm to an unborn baby.
The common side effects that occurred in more than a half of patients who received DANYELZA were infusion-related reactions, pain, fast heart beats, vomiting, cough, nausea, decreased appetite, and diarrhea.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse events in the clinical trials.
Table 5. Adverse Events (>10%) in Patients with Refractory or Relapsed High-Risk Neuroblastoma in Bone or Bone Marrow Who Received DANYELZA with GM-CSF in Trial 1
|
|
DANYELZA with GM-CSF1 |
|
|---|---|---|
|
All Grades |
Grade 3 or 4 |
|
|
Body system |
||
|
General disorders and administration site conditions |
||
|
Pain2 |
100 |
72 |
|
Infusion-related reaction3 |
100 |
68 |
|
Edema |
28 |
0 |
|
Fatigue4 |
28 |
0 |
|
Pyrexia5 |
28 |
0 |
|
Respiratory, thoracic and mediastinal disorders |
||
|
Cough |
60 |
0 |
|
Rhinorrhea |
24 |
0 |
|
Vascular disorders |
||
|
Hypertension |
44 |
4 |
|
Gastrointestinal disorders |
||
|
Vomiting |
60 |
4 |
|
Diarrhea |
56 |
8 |
|
Nausea |
56 |
0 |
|
Skin and subcutaneous tissue disorders |
||
|
Urticaria7 |
32 |
4 |
|
Cardiac disorders |
||
|
Tachycardia6 |
84 |
4 |
|
Nervous system disorders |
||
|
Peripheral neuropathy8 |
32 |
0 |
|
Headache |
28 |
8 |
|
Depressed level of consciousness |
24 |
16 |
|
Eye disorders |
||
|
Neurological disorders of the eye9 |
24 |
0 |
|
Immune system disorders |
||
|
Anaphylactic reaction |
12 |
12 |
|
Metabolism and nutrition disorders |
||
|
Decreased appetite |
16 |
0 |
|
Infections and infestations |
||
|
Influenza |
12 |
0 |
|
Rhinovirus infection |
12 |
0 |
|
Upper respiratory tract infection |
12 |
0 |
|
Investigations |
||
|
Weight decreased |
12 |
0 |
|
Psychiatric disorders |
||
|
Anxiety |
12 |
0 |
1Adverse events were graded using CTCAE version 4.0.
2Infusion-related reaction includes , bronchospasm, flushing, wheezing, stridor, urticaria, dyspnea, pyrexia, infusion related reaction, face edema, swelling face, tongue edema, lip edema, respiratory tract edema, angioedema, chills, hypoxia, pruritis, and respiratory distress occurring on the day of infusion or the day following an infusion.
3Pain includes pain, abdominal pain, pain in extremity, bone pain, neck pain, back pain, non-cardiac chest pain, flank pain, and musculoskeletal pain.
4Fatigue contains fatigue, asthenia.
5Pyrexia not occuring on the day of infusion or the day following an infusion.
6Tachycardia containsincludessinus tachycardia and tachycardia.
7Urticaria, not occurring on the day of infusion or the day following an infusion.
8Peripheral neuropathy includes peripheral sensory neuropathy, paresthesia, neuralgia.
9Neurological disorders of the eye includes unequal pupils, blurred vision, and mydriasis.
Table 6. Adverse Events (>10%) in Patients with Refractory or Relapsed High-Risk Neuroblastoma in Bone or Bone Marrow Who Received DANYELZA with GM-CSF in Trial 2
|
Adverse Event |
DANYELZA with GM-CSF1,2 |
|
|---|---|---|
|
All Grades |
Grade 3 or 4 |
|
|
Body system |
|
|
|
General disorders and administration site conditions |
||
|
Infusion-related reaction3 |
94 |
32 |
|
Pain4 |
94 |
2.8 |
|
Fatigue5 |
44 |
0 |
|
Injection site reaction |
28 |
0 |
|
Localized edema |
25 |
0 |
|
Pyrexia6 |
11 |
0 |
|
Vascular disorders |
||
|
Hypertension |
28 |
7 |
|
Gastrointestinal disorders |
||
|
Vomiting |
63 |
2.8 |
|
Nausea |
57 |
1.4 |
|
Diarrhea |
50 |
4.2 |
|
Constipation |
15 |
0 |
|
Skin and subcutaneous tissue disorders |
||
|
Erythema multiforme |
33 |
0 |
|
Hyperhidrosis |
17 |
0 |
|
Erythema |
11 |
0 |
|
Respiratory, thoracic and mediastinal disorders |
||
|
Cough |
57 |
0 |
|
Oropharyngeal pain |
15 |
0 |
|
Rhinorrhea |
15 |
0 |
|
Nervous system disorders |
||
|
Peripheral neuropathy7 |
25 |
0 |
|
Headache |
18 |
0 |
|
Lethargy |
14 |
0 |
|
Metabolism and nutrition disorders |
||
|
Decreased appetite |
53 |
4.2 |
|
Cardiac disorders |
||
|
Sinus tachycardia |
44 |
1.4 |
|
Psychiatric disorders |
||
|
Anxiety |
26 |
0 |
|
Irritability |
25 |
0 |
|
Investigations |
||
|
Breath sounds abnormal |
15 |
0 |
|
Injury and procedural complications |
||
|
Contusion |
15 |
0 |
|
Infections and infestations |
||
|
Rhinovirus infection |
14 |
0 |
|
Enterovirus infection |
13 |
0 |
|
Eye Disorders |
||
|
Neurological disorders of the eye8 |
19 |
0 |
1In Trial 2 all adverse events occurring in Cycle 1 and 2, and adverse events of ≥ Grade 3 severity occurring in subsequent cycles were reported. In the dose finding phase, additionally Grade 2 unexpected adverse reactions (adverse events which are considered possibly, probably, or definitely related according to protocol) were also reported for Cycles 3 and later.
2Adverse events were graded using CTCAE version 4.0.
3Infusion-related reaction includes bronchospasm, flushing, wheezing, stridor, urticaria, dyspnea, pyrexia, face edema, swelling face, periorbital edema, lip swelling, swollen tongue, respiratory tract edema, chills, hypoxia, pruritis, and erythema multiforme occurring on the day of infusion or the day following an infusion.
4Pain includes pain, abdominal pain, pain in extremity, bone pain, neck pain, back pain, non-cardiac chest pain, flank pain, and musculoskeletal pain.
5Fatigue includes fatigue, asthenia.
6Pyrexia not occurring on the day of infusion or the day following an infusion.
7Peripheral neuropathy includes peripheral sensory neuropathy, peripheral motor neuropathy, paresthesia, neuralgia.
8Neurological disorders of the eye contains unequal pupils, blurred vision, accommodation disorder, visual impairment, photophobia and mydriasis.
DANYELZA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurence of side effects were similar in males and females.
- Race: The majority of patients were White. Therefore, differences in side effects among races could not be determined.
- Age: The majority of patients were 12 years or younger. Therefore, differences in side effects among patients of different age groups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The following tables summarize adverse events by demographic subgroups in individual trials. Given the small sample size for the subgroups, results must be interpreted with caution.
Table 7. Subgroup Analysis of Adverse Events by Sex
|
|
Trial 1 |
Trial 2 |
||
|---|---|---|---|---|
|
Male |
Female |
Male |
Female |
|
|
Patients with at least one TEAE |
16 (100%) |
9 (100%) |
45 (100%) |
27 (100%) |
|
Patients with at least one TEAE of grade 3 or higher |
15 ( 94%) |
8 ( 89%) |
30 (67%) |
15 (56%) |
|
Patients with at least one SAE |
4 ( 25%) |
4 ( 44%) |
13 (29%) |
7 (26%) |
Table 8. Subgroup Analysis of Adverse Events by Race
|
|
Trial 1 |
Trial 2 |
||
|---|---|---|---|---|
|
White |
Asian |
White |
Asian |
|
|
Patients with at least one TEAE |
12 (100%) |
12 (100%) |
55 (100%) |
5 (100%) |
|
Patients with at least one TEAE of grade 3 or higher |
10 (83%) |
12 (100%) |
43 (89%) |
4 (80%) |
|
Patients with at least one SAE |
4 (33%) |
3 (25%) |
23 (42%) |
2 (40%) |
Table 9. Subgroup Analysis of Adverse Events by Age
|
|
Trial 1 |
Trial 2 |
|||
|---|---|---|---|---|---|
|
<12y |
≥12y |
<12 y |
12-17 y |
≥18 y |
|
|
Patients with at least one TEAE |
25 (100%) |
- |
65 (100%) |
5 (100%) |
2 (100%) |
|
Patients with at least one TEAE of grade 3 or higher |
23 (92%) |
- |
58 (89% |
4 (80%) |
2 (100%) |
|
Patients with at least one SAE |
8 (32%) |
- |
17 (26%) |
2 (40%) |
1 (50%) |
TEAE=treatment-emergent adverse event;SAE=serious adverse event
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved DANYELZA based on two clinical trials (Trial 1/NCT03363373 and Trial 2/NCT01757626) 97 patients with high-risk neuroblastoma in bone or bone marrow. The trials were conducted at 4 centers in the United States and in Spain.
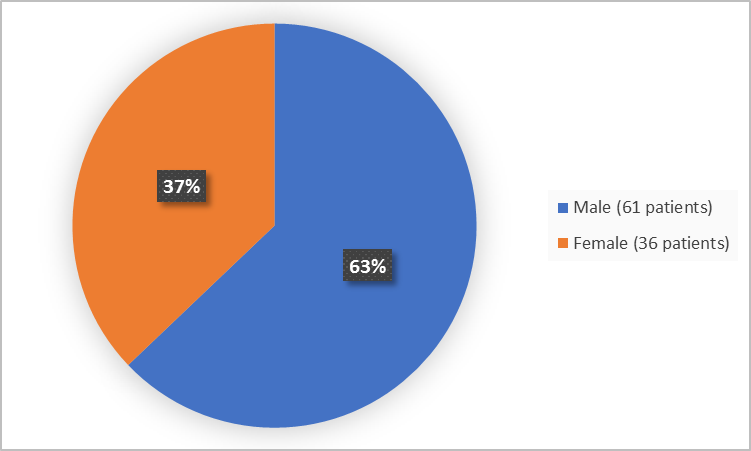
Figure 1 summarizes how many boys and girls were in the clinical trials.
Figure 1. Demographics by Sex
Adapted from FDA Review
Figure 2 summarizes by race how many patients were in the clinical trials.
Figure 2. Demographics by Race
Adapted from FDA Review
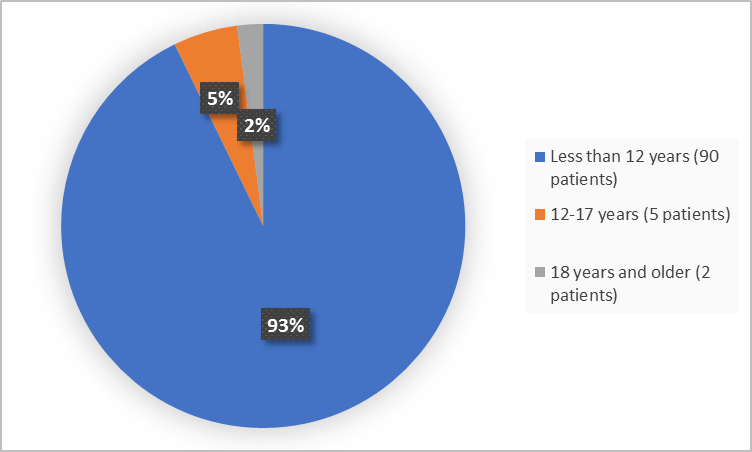
The figure below summarizes the age groups of patients in the clinical trials.
Figure 3. Demographics by Age
Adapted from FDA Review
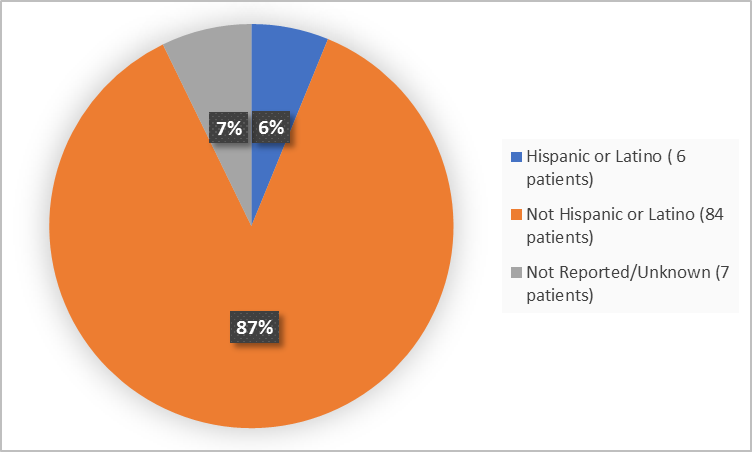
The figure below summarizes the patients by ethnicity in the clinical trials.
Figure 4. Demographics by Ethnicity
Adapted from FDA Review
Who participated in the trials?
The table below summarizes demographics for the trials that supported the approval of DANYELZA.
Table 10. Trials Demographics (safety population)
|
Demographic Parameters |
Trial 1 |
Trial 2 |
TOTAL |
|---|---|---|---|
|
Sex |
|||
|
Male |
16 (64) |
45 (63) |
61 (63) |
|
Female |
9 (36) |
27 (38) |
36 (37) |
|
Race |
|||
|
White |
12 (48) |
55 (76) |
67 (69) |
|
Black or African American |
0 |
3 (4) |
3 (3) |
|
Asian |
12 (48) |
5 (7) |
17 (18) |
|
American Indian or Alaska Native |
0 |
2 (3) |
2 (2) |
|
Other |
1 (4) |
2 (3) |
3 (3) |
|
Not Reported |
0 |
5 (7) |
5 (5) |
|
Age |
|||
|
Median (years) |
5 |
5 |
5 |
|
Min, max (years) |
1,10 |
2, 24 |
1, 24 |
|
Age Group |
|||
|
<12 years |
25 (100) |
65 (90) |
90 (93) |
|
12 - 17 years |
0 |
5 (7) |
5 (5) |
|
≥ 18 years |
0 |
2 (3) |
2 (2) |
|
Ethnicity |
|||
|
Hispanic or Latino |
2 (8) |
4 (6) |
6 (6) |
|
Not Hispanic or Latino |
19 (76) |
65 (90) |
84 (87) |
|
Not Reported/Unknown (4) |
4 (16) |
3 (4) |
7 (7) |
|
Region |
|||
|
United States |
9 (36) |
72 (100) |
81 (84) |
|
Spain |
16 (74) |
0 |
16 (16) |
Adapted from FDA Review
How were the trials designed?
Both trials enrolled patients who were previously treated for high-risk neuroblastoma in the bone or bone marrow. Some patients were not responding to the previous therapies anymore and some patients experienced the return of the cancer. Patients with cancer that was actively growing after their last therapy were not included in the trial. All patients received DANYELZA in combination with GM-CSF according to the trial schedule.
The trials measured the percent of patients whose tumors completely or partially shrank, and whose bone marrow disease partially or completely responded, after treatment (overall response rate) and how long that shrinkage lasted.
How were the trials designed?
The safety and effectiveness of DANYELZA were evaluated in two open-label, single arm trials in patients with high-risk neuroblastoma in bone or bone marrow with refractory or relapsed disease.
Patients received DANYELZA 9 mg/kg/cycle administered as three separate intravenous infusions of 3 mg/kg (on Days 1, 3 and 5) in the first week of each cycle and GM-CSF subcutaneously at 250 µg/m2/day on Days -4 to 0 and at 500 µg/m2/day on Days 1 to 5.
The major efficacy outcome measure was overall response rate (ORR) as determined by independent pathology and imaging review according to the revised International Neuroblastoma Response Criteria (INRC) and duration of response (DOR), as determined by independent pathology and imaging review.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.