Drug Trials Snapshots: ZEJULA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to ZEJULA Prescribing Information for complete information.
ZEJULA (niraparib)
zuh-JOO-luh
Tesaro, Inc.
Approval date: March 27, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ZEJULA is a drug used for the maintenance treatment of adult patients with recurrent ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. It is to be used in patients who have responded to their most recent platinum-based chemotherapy regimen with either complete or partial tumor shrinkage.
How is this drug used?
Three capsules (total of 300 mg) are taken once a day with or without food.
What are the benefits of this drug?
In the trial, women taking ZEJULA experienced a longer time period before their tumors worsened, in comparison to women who took placebo. Information on overall survival of these women is not available at this time.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the clinical trial based on the Intent-To-Treat (ITT) population and presented by germline BRCA gene mutation status (gBRCAmut). Primary endpoint was Progression-Free Survival (PFS) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1
Table 2. Efficacy Results - Trial 1 (IRC Assessmenta, Intent-To-Treat Population)
| gBRCAmut Cohort | non-gBRCAmut Cohort | |||
|---|---|---|---|---|
| ZEJULA (N=138) | Placebo (N=65) | ZEJULA (N=234) | Placebo (N=116) | |
| PFS Median in months (95% CI) | 21.0 (12.9, NR) | 5.5 (3.8, 7.2) | 9.3 (7.2, 11.2) | 3.9 (3.7, 5.5) |
| Hazard Ratio (HR)b (95% CI) | 0.26 (0.17, 0.41) | 0.45 (0.34, 0.61) | ||
| p-valuec | > | > | ||
a efficacy analysis was based on blinded central independent radiologic and clinical oncology review committee (IRC).
b based on a stratified Cox proportional hazards model
c based on a stratified log-rank test
NR=Not Reached
ZEJULA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
All the patients in the trial were women, therefore only race and age differences were analyzed.
- Race: The majority of patients in the clinical trials were White. Differences in response to ZEJULA among races could not be determined.
- Age: ZEJULA worked similarly in patients above and below 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results of IRC-PFS by age subgroup.
Table 3. Efficacy Analysis by Age Group
| ZEJULA | Placebo | IRC-PFS HR (95% CI) | |||
|---|---|---|---|---|---|
| n of events/ N of patients | Median (months) | n of events/ N of patients | Median (months) | ||
| gBRCAmut cohort | |||||
| Age Group (years) | |||||
| > | 47/110 | 15.5 | 32/49 | 7.2 | 0.32 (0.20, 0.51) |
| ≥65 | 11/28 | NR | 12/16 | 3.7 | 0.23 (0.10, 0.55) |
| Non-gBRCAmut cohort | |||||
| Age Group (years) | |||||
| > | 75/130 | 7.4 | 52/69 | 4.2 | 0.58 (0.40, 0.83) |
| ≥65 | 50/104 | 11.1 | 36/47 | 3.8 | 0.40 (0.26, 0.62) |
Adapted from FDA Review
What are the possible side effects?
ZEJULA may cause serious side effects including a bone marrow disorder (myelodysplastic syndrome), leukemia, and life-threatening high blood pressure.
Some of the most common side effects of ZEJULA are low blood cell counts (red, white and platelets), nausea, tiredness, constipation, vomiting, and stomach-area pain.
What are the possible side effects (results of trials used to assess safety)
The tables below summarize adverse reactions and electrolyte abnormalities in the clinical trial based on safety population defined as all patients who received at least one dose of the trial drug.
Table 4. Adverse Reactions Reported in ≥10% of Patients Receiving ZEJULA
| Grades 1-4* | Grades 3-4* | |||
|---|---|---|---|---|
| Zejula N=367 % | Placebo N=179 % | Zejula N=367 % | Placebo N=179 % | |
| Blood and lymphatic system disorders | ||||
| Thrombocytopenia | 61 | 5 | 29 | 0.6 |
| Anemia | 50 | 7 | 25 | 0 |
| Neutropenia | 30 | 6 | 20 | 2 |
| Leukopenia | 17 | 8 | 5 | 0 |
| Cardiac Disorders | ||||
| Palpitations | 10 | 2 | 0 | 0 |
| Gastrointestinal Disorders | ||||
| Nausea | 74 | 35 | 3 | 1 |
| Constipation | 40 | 20 | 0.8 | 2 |
| Vomiting | 34 | 16 | 2 | 0.6 |
| Abdominal pain/distention | 33 | 39 | 2 | 2 |
| Mucositis/stomatitis | 20 | 6 | 0.5 | 0 |
| Diarrhea | 20 | 21 | 0.3 | 1 |
| Dyspepsia | 18 | 12 | 0 | 0 |
| Dry mouth | 10 | 4 | 0.3 | 0 |
| General disorders and Administration Site Conditions | ||||
| Fatigue/Asthenia | 57 | 41 | 8 | 0.6 |
| Metabolism and Nutrition Disorders | ||||
| Decreased appetite | 25 | 15 | 0.3 | 0.6 |
| Infections and Infestations | ||||
| Urinary tract infection | 13 | 8 | 0.8 | 1 |
| Investigations | ||||
| AST/ ALT elevation | 10 | 5 | 4 | 2 |
| Musculoskeletal and Connective Tissue Disorders | ||||
| Myalgia | 19 | 20 | 0.8 | 0.6 |
| Back pain | 18 | 12 | 0.8 | 0 |
| Arthralgia | 13 | 15 | 0.3 | 0.6 |
| Nervous system Disorders | ||||
| Headache | 26 | 11 | 0.3 | 0 |
| Dizziness | 18 | 8 | 0 | 0 |
| Dysgeusia | 10 | 4 | 0 | 0 |
| Psychiatric Disorders | ||||
| Insomnia | 27 | 8 | 0.3 | 0 |
| Anxiety | 11 | 7 | 0.3 | 0.6 |
| Respiratory, Thoracic, and Mediastinal Disorders | ||||
| Nasopharyngitis | 23 | 14 | 0 | 0 |
| Dyspnea | 20 | 8 | 1 | 1 |
| Cough | 16 | 5 | 0 | 0 |
| Skin and Subcutaneous Tissue Disorders | ||||
| Rash | 21 | 9 | 0.5 | 0 |
| Vascular Disorders | ||||
| Hypertension | 20 | 5 | 9 | 2 |
*CTCAE=Common Terminology Criteria for Adverse Events version 4.02
ZEJULA Prescribing Information
Table 5. Abnormal Laboratory Findings in ≥25% of Patients Receiving ZEJULA
| Grades 1-4 | Grades 3-4 | |||
|---|---|---|---|---|
| ZEJULA N=367 (%) | Placebo N= 179 (%) | ZEJULA N= 367 (%) | Placebo N= 179 (%) | |
| Decrease in hemoglobin | 85 | 56 | 25 | 0.5 |
| Decrease in platelet count | 72 | 21 | 35 | 0.5 |
| Decrease in WBC count | 66 | 37 | 7 | 0.7 |
| Decrease in absolute neutrophil count | 53 | 25 | 21 | 2 |
| Increase in AST | 36 | 23 | 1 | 0 |
| Increase in ALT | 28 | 15 | 1 | 2 |
WBC=white blood cells; ALT=Alanine aminotransferase; AST=Aspartate aminotransferase
ZEJULA Prescribing Information
Were there any differences in side effects among sex, race and age?
All the patients in the trial were women, therefore only race and age differences were analyzed.
- Race: The majority patients in the clinical trials were White. Differences in side effects among races could not be determined.
- Age: The occurrence of side effect was similar between patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events Grade 1- 4 (G1-4) during the clinical trial by age subgroup.
Table 5. Subgroup Analysis of Adverse Events by Age Group
| Common G1-4 Adverse Events by Preferred Term | ZELUJA n=367 | ||
|---|---|---|---|
| Age 50> N= 55 (%) | Age 50- 65> N= 183 (%) | Age ≥ 65 N= 129 (%) | |
| Anemia | 25 (45) | 92 (50) | 65 (50) |
| Thrombocytopenia | 33 (60) | 81 (44) | 85 (66) |
| Neutropenia | 18 (33) | 59 (32) | 34 (26) |
| Nausea | 41 (75) | 143 (78) | 86 (66) |
| Constipation | 19 (35) | 73 (40) | 57 (44) |
| Abdominal pain | 15 (27) | 77 (42) | 44 (34) |
| Vomiting | 23 (42) | 68 (37) | 35 (27) |
| Diarrhea | 15 (27) | 37 (20) | 19 (15) |
| Decreased appetite | 11 (20) | 39 (21) | 43 (33) |
| Fatigue/ Asthenia | 32 (58) | 116 (63) | 71 (55) |
| Headache | 18 (33) | 54 (30) | 28 (22) |
| Insomnia | 9 (16) | 49 (27) | 33 (26) |
| Anxiety | 2 (4) | 18 (10) | 11 (9) |
| Hypertension | 7 (13) | 53 (29) | 26 (20) |
Adapted from FDA Clinical review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The benefit and side effects of ZEJULA were evaluated based on evidence from 553 women with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer.
The trial was conducted in the USA, Canada, Europe, and Israel.
Figure 1 summarizes how many women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
Adapted from FDA review
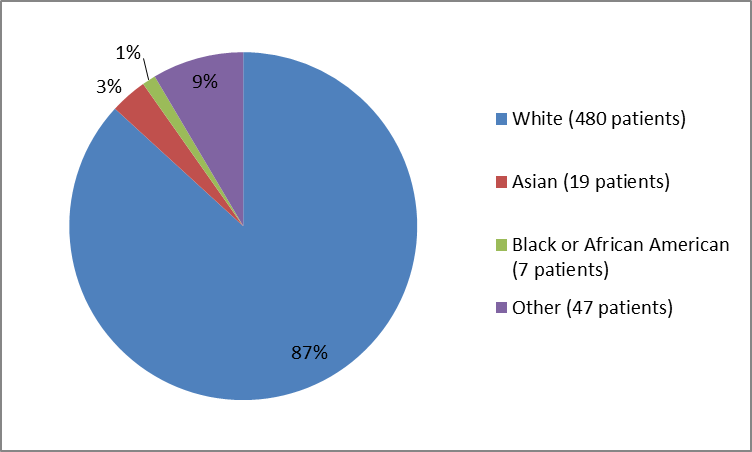
Figure 2 and Table 1 below summarize the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
Adapted from FDA review
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 480 | 87 |
| Asian | 19 | 3 |
| Black or African American | 7 | 1 |
| American Indian or Alaska Native | 1 | less than 1 |
| Unknown | 46 | 9 |
Adapted from FDA review
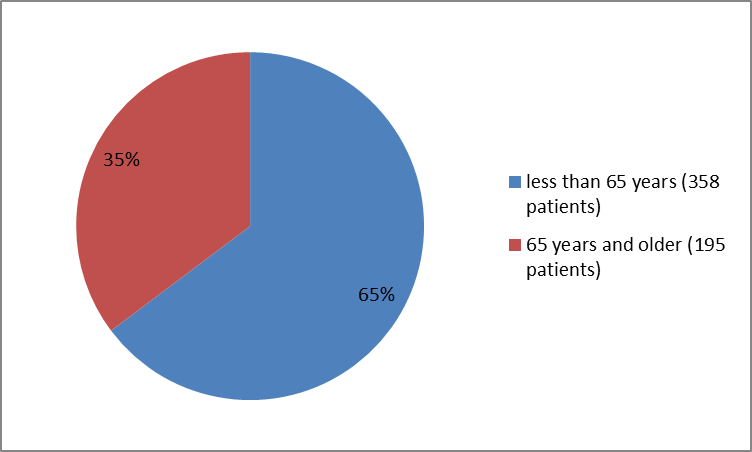
Figure 3 below summarizes the percentage of patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age
Adapted from FDA review
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trial.
Table 6. Baseline Demographics of Patients in the Clinical Trial (ITT population)
| Demographic | gBRCAmut cohort N= 203 | Non-gBRCAmut cohort N=350 | ||
|---|---|---|---|---|
| ZEJULA N=138 n(%) | Placebo N= 65 n(%) | ZEJULA N=234 n(%) | Placebo N=116 n(%) | |
| Age (years) | ||||
| Mean (SD) | 56.9 (9.3) | 57.2 (9.2) | 62 (9.4) | 59 (9.6) |
| Median | 57 | 58 | 63 | 60 |
| Range | 36,83 | 38, 73 | 33, 84 | 34, 82 |
| Age Group (years) | ||||
| 18-64 | 110 (80) | 49 (75) | 130 (56) | 69 (59) |
| ≥ 65 | 28 (20) | 16 (25) | 104 (44) | 47 (41) |
| ≥ 75 | 4 (3) | 0 | 19 (8) | 16 (14) |
| Race | ||||
| White | 123 (89) | 55 (84) | 201 (86) | 101 (87) |
| Black or African American | 1 (0.7) | 1 (2) | 4 (2) | 1 (1) |
| Asian | 2 (1.4) | 3 (6) | 10 (4) | 4 (3) |
| American Indian/ Alaska Native | 1 (0.7) | 0 | 0 | 0 |
| Unknown | 11 (8) | 6 (8) | 19 (8) | 10 (9) |
| Geographic Region | ||||
| US, Canada | 53 (38) | 28 (43) | 96 (41) | 44 (28) |
| Europe, Israel | 53 (38) | 28 (43) | 96 (41) | 44 (28) |
Adapted from FDA review
How were the trials designed?
The benefit and side effects of ZEJULA were evaluated in one clinical trial of women with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer. All patients had received at least two prior platinum regimens and had responded most recently (within 8 months) to a platinum-based therapy with either complete or partial tumor shrinkage.
Patients were tested with an FDA-approved test to determine whether they had a specific gene mutation, called a deleterious or germline BRCA mutation and then received either ZEJULA or placebo capsules once a day. Neither the patients nor the health care providers knew which treatment was being given until the trials were completed. The treatment continued until the disease progressed or the side effects became too toxic.
The benefit of ZEJULA was evaluated by measuring the duration of time in each group before the tumors worsened.
How were the trials designed?
There was one double-blind, placebo-controlled trial of women with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer within 8 weeks of the last therapy. All patients were in complete or partial response to their most recent platinum-based regimen and assigned to one of two cohorts based on the test results of the germline BRCA mutation.
The efficacy outcome measures was progression-free survival (PFS) assessed by the central independent radiologic and clinical oncology review committee (IRC) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.