Drug Trials Snapshots: ZEGALOGUE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the ZEGALOGUE Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
ZEGALOGUE (dasiglucagon)
(Ze’ gah log)
Zealand Pharma US, Inc.
Approval date: March 22, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ZEGALOGUE is a prescription medicine used to treat very low blood sugar (severe hypoglycemia) in people with diabetes aged 6 years and older.
How is this drug used?
ZEGALOGUE is injected under the skin (subcutaneously) in the lower abdomen, buttocks, thigh, or outer upper arm by a caregiver to a person with diabetes who has very low blood sugar (glucose). It is available in prefilled syringe and autoinjector devices, and each device contains a single dose (0.6 mg) of ZEGALOGUE. If there is no response after 15 minutes, another dose of ZEGALOGUE may be given using a new device.
What are the benefits of this drug?
In clinical trials, ZEGALOGUE was better than placebo at increasing blood sugar levels after a single injection in people with type 1 diabetes.
Who participated in the clinical trials?
The FDA approved ZEGALOGUE based on evidence from two clinical trials conducted in adult patients with type 1 diabetes (169 patients) and one clinical trial conducted in pediatric patients older than 6 years with type 1 diabetes (31 patients). The trials were conducted in the following five countries: United States, Germany, Austria, Canada, and Slovenia. The same trials were used to assess the safety and efficacy of ZEGALOGUE: adult Trials A and B and pediatric Trial C.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 summarizes efficacy results from two, placebo-controlled trials (Trial A and Trial B) conducted in adult patients with type 1 diabetes. The primary endpoint was time to plasma glucose recovery (treatment success), defined as an increase in blood glucose of ≥20 mg/dL from the time of administration, without additional intervention within 45 minutes. Plasma glucose values were collected and recorded at baseline (pre-dose), and at 4, 6, 8, 10, 12, 15, 17, 20, 25, 30, 40, and 45 minutes after treatment. Any patient who had not recovered by 45 minutes was censored at 45 minutes. Because times to plasma glucose recovery were monitored only periodically, the recorded time to recovery and consequently the estimated median time to recovery were not on a continuum but an interval scale. For example, for a given subject, a recorded time to recovery of 10 minutes means their actual time to recovery was between 8 and 10 minutes. An estimated median time to recovery of 10 minutes implies the actual median time to recovery falls somewhere between 8 and 10 minutes, the corresponding 95% confidence interval of (10, 10) implies the actual lower 95% confidence limit for the median time to recovery is between 8 to 10 minutes, and the upper 95% confidence limit is 10 minutes. Of note, upper limit of 95% CI can be unlimited (open-ended) due to period monitoring of plasma glucose within a certain monitoring time frame.
The median time to plasma glucose recovery was statistically significantly shorter for ZEGALOGUE (10 minutes) versus placebo (35 to 40 minutes).
Table 1. Plasma Glucose Recovery in Adult Patients (Trials A and B)
| Parameter | Trial A | Trial B | ||
|---|---|---|---|---|
| ZEGALOGUE N=82 |
Placebo N=43 |
ZEGALOGUE N=34 |
Placebo N=10 |
|
| Median time to recovery, minutes (95% CIa) | 10 (10, 10)b | 40 (30, 40) | 10 (8, 12)b | 35 (20, ∞)c |
Source: Adapted from FDA Review
a log-log confidence interval
b p<0.001 versus placebo (log-rank test stratified by injection sites)
c ∞: Upper limit of 95% CI is unlimited due to periodic monitoring of plasma glucose.
Abbreviations: CI, confidence interval; N, the number of patients who were randomized and treated.
Table 2 summarizes the efficacy results from one placebo-controlled study in pediatric patients (Trial C) aged 6 years and older with type 1 diabetes. This trial had the same primary endpoint as the clinical trials conducted in adult patients (above). The median time to plasma glucose recovery was statistically significantly shorter for ZEGALOGUE (10 minutes) versus placebo (30 minutes).
Table 2. Plasma Glucose Recovery in Pediatric Patients (Trial C)
| Parameter | Trial C | |
|---|---|---|
| ZEGALOGUE N=20 |
Placebo N=11 |
|
| Median timea to recovery, minutes (95% CIb) | 10 (8, 12)c | 30 (20; ∞)d |
Source: Adapted from FDA Review
a Refer to detailed explanation above Table 1 on how to interpret the estimated median time to plasma glucose recovery (95% CI) in this table
b log-log confidence interval
c p<0.001 versus placebo (log-rank test stratified by injection site and age group)
d ∞: Upper limit of 95% CI is unlimited due to periodic monitoring of plasma glucose.
Abbreviations: CI, confidence interval; N, the number of patients who were randomized and treated.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ZEGALOGUE worked similarly in male and female patients.
- Race: The number of patients of races other than White was small; therefore, differences in how ZEGALOGUE worked among races could not be determined.
- Age: The number of patients 65 years old or older was small; therefore, differences in how well the drug worked between older adults and younger adults could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
As shown in Table 3, ZEGALOGUE resulted in a faster time to plasma glucose recovery as compared to placebo in adult patients with type 1 diabetes (Trials A and B). Superiority of ZEGALOGUE was demonstrated in all subgroups.
Table 3. Time to Plasma Glucose Recovery by Subgroups in Adult Patients (Trials A and B)
| Demographic Parameters | ZEGALOGUE | Placebo | ||
|---|---|---|---|---|
| N (n) | Median Timea to Plasma Glucose Recovery, Minutes (95% CIb) | N (n) | Median Timea to Plasma Glucose Recovery, Minutes (95% CIb) | |
| Overall | 116 (115) | 10 (10, 10) | 53 (38) | 40 (30, 40) |
| Sex | ||||
| Male | 66 (66) | 10 (10, 12) | 36 (23) | 40 (30, ∞)c |
| Female | 50 (49) | 10 (8, 10) | 17 (15) | 30 (20, 40) |
| Age, years | ||||
| <65 | 111 (110) | 10 (10, 10) | 52 (37) | 40 (30, 40) |
| ≥65 | 5 (5) | 15 (15, ∞)c | 1 (1) | 30 (0, ∞)c |
| Q1: <30 | 26 (26) | 8 (8, 10) | 16 (15) | 25 (25, 30) |
| Q2: ≥30 to <37 | 26 (26) | 10 (10, 10) | 13 (7) | 45 (30, ∞)c |
| Q3: ≥37 to <48 | 30 (30) | 10 (8, 12) | 10 (9) | 40 (20, 40) |
| Q4: ≥48 | 34 (33) | 12 (10,12) | 14 (7) | 40 (30, ∞)c |
| Race | ||||
| White | 110 (109) | 10 (10, 10) | 46 (35) | 35 (30, 40) |
| Black or African American | 1 (1) | 12 (0, ∞)c | 2 (2) | 37.5 (30, ∞)c |
| Others | 5 (5) | 10 (8, ∞)c | 5 (1) | 20 (0, ∞)c |
Source: Adapted from FDA
a Refer to detailed explanation above Table 1 on how to interpret the estimated median time to plasma glucose recovery (95% CI) in this table
b log-log confidence interval
c ∞: Upper limit of 95% CI is unlimited due to periodic monitoring of plasma glucose.
Abbreviations: CI, confidence interval; N, the number of patients who were randomized and treated; n, the number of subjects in subgroup who recovered within 45 minutes after dosing without a rescue intravenous (IV) glucose administration; Q1, first age quartile; Q2, second age quartile; Q3, third age quartile; Q4, fourth age quartile
As shown in Table 4, ZEGALOGUE resulted in a faster time to plasma glucose recovery as compared to placebo in pediatrics with type 1 diabetes (Trial C). Superiority of ZEGALOGUE was demonstrated in all subgroups. In Trial C, all subjects except 2 subjects were white in race, and therefore a subgroup analysis for race was not performed.
Table 4. Time to Plasma Glucose Recovery by Subgroups in Pediatric Patients (Trial C)
| Demographic Parameters | ZEGALOGUE | Placebo | ||
|---|---|---|---|---|
| N (n) | Median Timea to Plasma Glucose Recovery, Minutes (95% CIb) | N (n) | Median Timea to Plasma Glucose Recovery, Minutes (95% CIb) | |
| Overall | 20 (20) | 10 (8, 12) | 11 (7) | 30 (20, ∞)c |
| Sex | ||||
| Male | 10 (10) | 10 (8, 12) | 5 (3) | 45 (30, ∞c) |
| Female | 10 (10) | 9 (8, 12) | 6 (4) | 30 (17, ∞)c |
| Age, years | ||||
| 6 to 11 | 8 (8) | 9 (8, 12) | 4 (3) | 25 (17, ∞)c |
| 12 to 17 | 12 (12) | 10 (8,12) | 7 (4) | 45 (30, ∞)c |
Source: Adapted from FDA Review
a Refer to detailed explanation above Table 1 on how to interpret the estimated median time to plasma glucose recovery (95% CI) in this table
blog-log confidence interval
c∞: Upper limit of 95% CI is unlimited due to periodic monitoring of plasma glucose.
Abbreviations: CI, confidence interval; N, the number of patients who were randomized and treated; n, the number of subjects in subgroup who recovered within 45 minutes after dosing without a rescue intravenous (IV) glucose administration.
What are the possible side effects?
The most common side effects of ZEGALOGUE are nausea, vomiting, headache, diarrhea, and injection site pain. ZEGALOGUE should not be used in people who have a specific type of tumor in their adrenal glands (pheochromocytoma) or pancreas (insulinoma). Allergic reactions, including anaphylactic shock with breathing difficulties and low blood pressure (hypotension) have been observed with similar products. ZEGALOGUE may not be effective in patients with insufficient sugar (glycogen) stores in the liver, which can be caused by starvation, adrenal insufficiency, and chronic low blood sugar (hypoglycemia).
What are the possible side effects (results of trials used to assess safety)?
The data in Table 5 reflect exposure of 116 adult patients with type 1 diabetes to ZEGALOGUE in two placebo-controlled trials (Trials A and B). Adverse reactions occurring in ≥2% and more frequently than with placebo within 12 hours of treatment are shown. Other adverse reactions observed in adult patients treated with ZEGALOGUE occurring within 12 hours of treatment include hypertension, hypotension, bradycardia, presyncope, palpitations, and orthostatic intolerance.
Table 5. Adverse Reactions Occurring in ≥2% and More Frequently Than With Placebo in ZEGALOGUE-Treated Adult Patients Within 12 Hours of Treatment in 2 Placebo-Controlled Trials
| Adverse Reaction | Placebo N=53 % of Patients |
ZEGALOGUE N=116 % of Patients |
|---|---|---|
| Nausea | 4 | 57 |
| Vomiting | 2 | 25 |
| Headache | 4 | 11 |
| Diarrhea | 0 | 5 |
| Injection site pain | 0 | 2 |
Source: Adapted from FDA Review
The data in Table 6 reflect exposure of 20 pediatric patients with type 1 diabetes to ZEGALOGUE in one placebo-controlled trial (Trial C). Adverse reactions occurring in ≥2% of patients and more frequently than with placebo within 12 hours of treatment are shown in older pediatric patients (12 to 17 years) and younger pediatric patients (6 to 11 years).
Table 6. Adverse Reactions Occurring in ≥2% and More Frequently Than With Placebo in ZEGALOGUE-Treated Pediatric Patients Within 12 hours of Treatment in a Placebo-Controlled Trial
| Adverse Reaction | Placebo N=11 % of Patients |
ZEGALOGUE Age 6 to 11 Years N=8 % of Patients |
ZEGALOGUE Age 12 to 17 Years N=12 % of Patients |
ZEGALOGUE All Ages N=20 % of Patients |
|---|---|---|---|---|
| Nausea | 0 | 25 | 92 | 65 |
| Vomiting | 0 | 25 | 67 | 50 |
| Headache | 0 | 0 | 17 | 10 |
| Injection site pain | 0 | 0 | 8 | 5 |
Source: Adapted from FDA Review
Were there any differences in side effects among sex, race and age?
- Sex: There were more gastrointestinal side effects (nausea, vomiting, diarrhea) in females than in males. Other side effects appeared similar between males and females.
- Race: The number of patients of races other than was small; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The number of patients 65 years old or older was small; therefore, differences in the occurrence of side effects between older adults and younger adults could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 7 shows the safety analyses by demographic subgroup for adverse events of nausea, vomiting, headache, and diarrhea.
Table 7. Safety Analysis by Demographic Subgroups in Adults Patients (Trials A and B)
| Demographic | N=169 % of Population |
Nausea | Vomiting | Headache | Diarrhea | ||||
|---|---|---|---|---|---|---|---|---|---|
| ZEGALOGUE n/N (%) |
Placebo n/N (%) |
ZEGALOGUE n/N (%) |
Placebo n/N (%) |
ZEGALOGUE n/N (%) |
Placebo n/N (%) |
ZEGALOGUE n/N (%) |
Placebo n/N (%) |
||
| Sex | |||||||||
| Male | 60 | 33/66 (50) | 1/36 (3) | 13/66 (20) | 0/36 (0) | 8/66 (12) | 0/36 (0) | 1/66 (2) | 0/36 (0) |
| Female | 40 | 33/50 (66) | 1/17 (6) | 16/50 (32) | 1/17 (6) | 5/50 (10) | 2/17 (12) | 5/50 (10) | 0/17 (0) |
| Age | |||||||||
| ≥18 to <65 | 96 | 65/111 (59) | 2/52 (4) | 29/111 (26) | 1/52 (2) | 12/111 (11) | 2/52 (4) | 5/111 (5) | 0/52 (0) |
| ≥65 to <75 | 4 | 1/5 (20) | 0/1 (0) | 0/5 (0) | 0/1 (0) | 1/5 (20) | 0/1 (0) | 1/5 (20) | 0/1 (0) |
| Race | |||||||||
| White | 92 | 63/110 (57) | 2/46 (4) | 27/110 (25) | 1/46 (2) | 13/110 (12) | 2/46 (4) | 6/110 (5) | 0/46 (0) |
| Black or African American | 2 | 1/1 (100) | 0/2 (0) | 0/1 (0) | 0/2 (0) | 0/1 (0) | 0/2 (0) | 0/1 (0) | 0/2 (0) |
| Asian | 3 | 1/3 (33) | 0/2 (0) | 1/3 (33) | 0/2 (0) | 0/3 (0) | 0/2 (0) | 0/3 (0) | 0/2 (0) |
| Other | 3 | 1/2 (50) | 0/3 (0) | 1/2 (50) | 0/3 (0) | 0/2 (0) | 0/3 (0) | 0/2 (0) | 0/3 (0) |
Source: Adapted from FDA Review
DEMOGRAPHICS SNAPSHOT
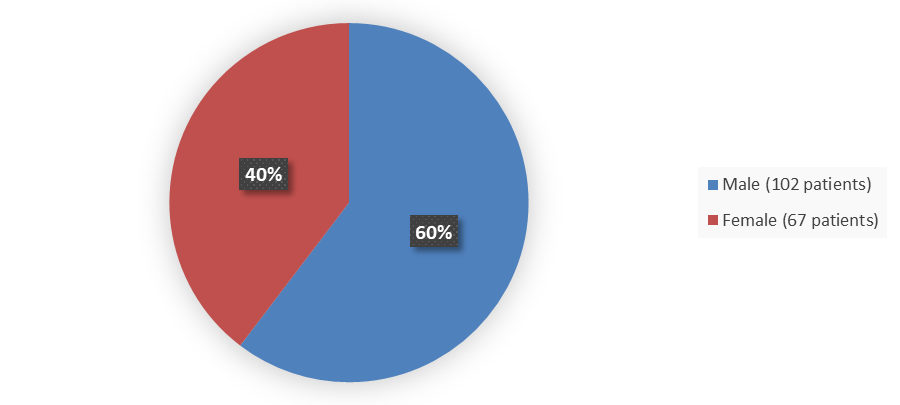
Figure 1 summarizes how many male and female patients were enrolled in the clinical trials used to evaluate the efficacy and side effects of ZEGALOGUE.
Figure 1. Baseline Demographics by Sex in Safety and Efficacy Population (Trials A and B)
Source: Adapted from FDA Review
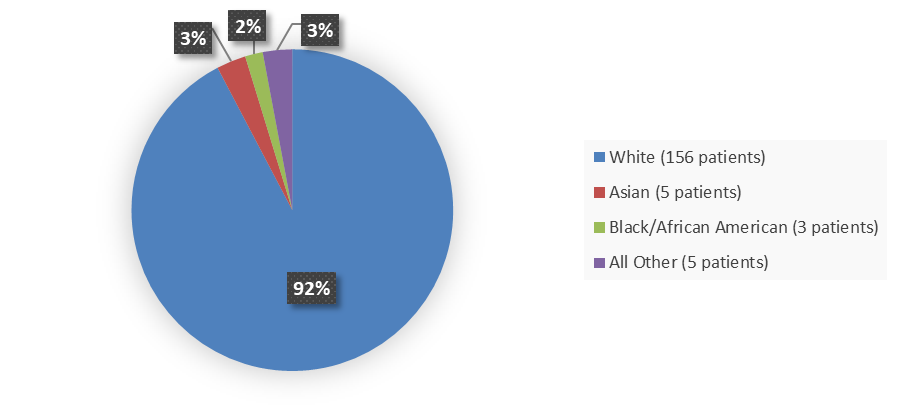
Figure 2 summarizes the percentage of patients by race were enrolled in the clinical trials used to evaluate the efficacy and side effects of ZEGALOGUE.
Figure 2. Baseline Demographics by Race in Safety and Efficacy Population (Trials A and B)
Source: Adapted from FDA Review
Figure 3 summarizes how many patients by age were enrolled in the clinical trials used to evaluate the efficacy and side effects of ZEGALOGUE.
Figure 3. Baseline Demographics by Age in Safety and Efficacy Population (Trials A and B)
Source: Adapted from FDA Review
Who participated in the trials?
Table 8 summarizes the baseline demographics of the combined adult clinical trials used to evaluate the safety and efficacy of ZEGALOGUE (Trials A and B).
Table 8. Demographics in Adult Patients With Type 1 Diabetes (Trials A and B)
| Demographic | ZEGALOGUE N=116 n (%) |
Placebo N=53 n (%) |
|---|---|---|
| Age, years | ||
| Mean (SD) | 40.1 (12.6) | 37.7 (13) |
| Median (min, max) | 38 (18, 71) | 35 (18, 65) |
| <65 | 111 (96) | 52 (98) |
| ≥65 | 5 (4) | 1 (2) |
| Sex | ||
| Male | 66 (57) | 36 (68) |
| Female | 50 (43) | 17 (32) |
| Race | ||
| White | 110 (93) | 46 (87) |
| Black or African American | 1 (1) | 2 (4) |
| Others | 5 (6) | 5 (9) |
Source: Adapted from FDA Review
Table 9 summarizes the baseline demographics of the pediatric clinical trial used to evaluate the efficacy and safety of ZEGALOGUE (Trial C).
Table 9. Demographics in Pediatric Patients With Type 1 Diabetes (Trial C)
| Demographic | ZEGALOGUE N=20 n (%) |
Placebo N=11 n (%) |
|---|---|---|
| Age, years | ||
| Mean (SD) | 12.3 (3.4) | 12.8 (3.3) |
| Min, max | 7, 17 | 7, 17 |
| 6 to 11 | 8 (40) | 4 (36) |
| 12 to 17 | 12 (60) | 7 (64) |
| Sex | ||
| Male | 10 (50) | 5 (46) |
| Female | 10 (50) | 6 (54) |
| Race | ||
| White | 19 (95) | 10 (91) |
| Others | 1 (5) | 1 (9 ) |
Source: Adapted from FDA Review
How were the trials designed?
There were three clinical trials that provided data for the approval of ZEGALOGUE.
Trials A and B were conducted in adults with type 1 diabetes and Trial C was conducted in children with type 1 diabetes 6 years of age and older.
In all trials, patients were randomized into one of three groups: one group received ZEGALOGUE, one group received placebo, and one group received glucagon for injection (a similar drug that is FDA approved for the same purpose as ZEGALOGUE). In all groups, patients were given insulin to decrease their blood sugar to a low level (hypoglycemia). Subjects received a single injection of either ZEGALOGUE, placebo, or glucagon for injection, and the ability of the treatment to increase the patients’ blood sugar was evaluated.
How were the trials designed?
Three randomized, double-blind, placebo-controlled, multicenter trials were conducted in patients with type 1 diabetes. Two trials (Trial A and Trial B) were conducted in adult patients, and one trial (Trial C) was conducted in pediatric patients aged 6 to 17 years. In all three trials, patients were randomized to ZEGALOGUE 0.6 mg, placebo, or (in Trials A and C) glucagon for injection 1.0 mg. ZEGALOGUE and the comparators were administered as single subcutaneous injections following a controlled induction of hypoglycemia using intravenous administration of insulin. During this procedure, a plasma glucose concentration of <60 mg/dL was targeted in Trials A and B, whereas the target was <80 mg/dL in Trial C.
The primary efficacy endpoint for all three trials was time to plasma glucose recovery (treatment success), defined as an increase in blood glucose of ≥20 mg/dL from time of administration, without additional intervention within 45 minutes. In Trials A and B, plasma glucose values were collected and assessed at pre-dose, and at 4, 6, 8, 10, 12, 15, 17, 20, 25, 30, 40, 45, 50, 60, 75, and 90 minutes after treatment. Trial C assessed plasma glucose at the same timepoints as did Trials A and B, with the exception of the 25, 40, 50, 75 and 90 minute post-treatment timepoints. Kaplan-Meier estimates for median time to recovery and corresponding 95% confidence interval were used to present the results in each group. Confidence interval was calculated using the intervals on the log hazard. The primary hypothesis test was superiority of ZEGALOGUE versus placebo. There was no formal hypothesis test of ZEGALOGUE versus glucagon for injection.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.