Drug Trials Snapshots: VIBERZI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the VIBERZI Prescribing Information for complete information.
VIBERZI (eluxadoline)
(vye BER zee)
Forest Pharmaceuticals, Inc.
Approval date: May 27, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VIBERZI is a treatment for adults with a disease of the gut called irritable bowel syndrome with diarrhea, commonly referred to as IBS-D.
How is this drug used?
VIBERZI is a tablet taken by mouth two times each day with food.
What are the benefits of this drug?
VIBERZI decreases abdominal pain and makes stool less watery.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the composite efficacy endpoint for each trial. This was based on the simultaneous improvement of Worst Abdominal Pain (WAP) by ≥30% and Bristol Stool Score (BSS) < 5="" on="" the="" same="" day="" for="" ≥50%="" of="" days="" over="" the="" 12="" weeks="" interval="" for="" viberzi="" 100="" mg="" and="" 75="" mg="" in="" comparison="" to="">
Table 2. Efficacy Results in Trials 1 and 2
| Trial 1 | Trial 2 | |||||
|---|---|---|---|---|---|---|
| Composite response* over 12 weeks | VIBERZI 100 mg n=426 |
VIBERZI 75 mg n=427 |
Placebo n=427 |
VIBERZI 100 mg n=382 |
VIBERZI 75 mg n=381 |
Placebo n=382 |
| Responder rates (%) | 25
|
24
|
17
|
30
|
29
|
16
|
| Treatment difference (%) | 8**
|
7****
|
-
|
13***
|
13***
|
-
|
| 95% CI (%) | (2.6,13.5)
|
(1.4,12.2)
|
-
|
(7.5,19.2)
|
(6.8, 18,5)
|
-
|
*Simultaneous improvement of Worst Abdominal Pain by ≥30% and Bristol Stool Score < 5="" on="" the="" same="" day="" for="" ≥50%="" of="" days="" over="" the="">
** p<>
*** p<>
****p<>
Source: Modified from VIBERZI Prescribing information, Section 14, Table 4
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The majority of patients in the trials were women. VIBERZI was similarly effective in men and women.
- Race: The majority of patients in the trials were white. Differences in response to VIBERZI between races could not be determined.
- Age: The majority of patients in the trials were less than 65 years of age. VIBERZI was similarly effective in all age groups studied.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize the results for the primary endpoint in the trial by subgroup.
Table 3. Subgroup Analysis of Primary Endpoint-Trial 1 (ITT population)
| Subgroup | VIBERZI 100 mg | VIBERZI 75 mg | Placebo | ||||
|---|---|---|---|---|---|---|---|
| x (%) | Total, n | x (%) | Total, n | x (%) | Total, n | ||
| Overall Response/All patients | 107 (25) | 426 | 102 (24) | 427 | 73 (17) | 427 | |
| Sex | |||||||
| Male | 31 (22) | 143 | 39 (26) | 151 | 20 (13) | 150 | |
| Female | 73 (27) | 283 | 63 (23) | 276 | 53 (19) | 277 | |
| Age Group | |||||||
| <65> | 95 (24) | 391 | 88 (22) | 398 | 66 (18) | 376 | |
| >= 65 years | 12 (34) | 35 | 14 (48) | 29 | 7 (14) | 51 | |
| Race | |||||||
| White | 94 (26) | 368 | 90 (24) | 372 | 67 (18) | 370 | |
| Black or African American | 9 (20) | 46 | 11 (24) | 46 | 6 (13) | 46 | |
| Other | 2 (20) | 10 | 1 (11) | 9 | 0 | 11 | |
| Ethnicity | |||||||
| Hispanic or Latino | 22 (19) | 117 | 18 (15) | 119 | 19 (15) | 125 | |
| Not Hispanic or Latino | 83 (27) | 307 | 84 (27) | 308 | 54 (18) | 302 | |
| Region | |||||||
| United States | 103 (26) | 404 | 94 (23) | 403 | 65 (16) | 405 | |
| Non US | 4 (18) | 22 | 8 (33) | 24 | 8 (36) | 22 | |
Source: Company Clinical Trial Data
Table 4. Subgroup Analysis of Primary Endpoint-Trial 2 (ITT population)
| Subgroup | VIBERZI 100 mg | VIBERZI 75 mg | Placebo | |||
|---|---|---|---|---|---|---|
| x (%) | Total, n | x (%) | Total, n | x (%) | Total, n | |
| Overall Response/All patients | 113 (30) | 382 | 110 (29) | 381 | 62 (16) | 382 |
| Sex | ||||||
| Male | 40 (32) | 126 | 35 (29) | 120 | 23 (17) | 132 |
| Female | 73 (29) | 256 | 75 (29) | 261 | 39 (16) | 250 |
| Age Group | ||||||
| <65> | 99 (29) | 343 | 94 (27) | 345 | 57(17) | 331 |
| >= 65 years | 14 (36) | 39 | 16 (44) | 36 | 5 (10) | 51 |
| Race | ||||||
| White | 99 (31) | 318 | 100 (31) | 327 | 53 (16) | 329 |
| Black or African American | 12 (24) | 50 | 10 (22) | 46 | 7 (16) | 43 |
| Other | 2 (14) | 14 | 0 | 8 | 2 (20) | 10 |
| Ethnicity | ||||||
| Hispanic or Latino | 30 (30) | 99 | 35 (36) | 98 | 17 (17) | 101 |
| Not Hispanic or Latino | 83 (29) | 283 | 75 (27) | 283 | 45 (16) | 281 |
| Region | ||||||
| United States | 106 (29) | 365 | 107 (29) | 366 | 61 (17) | 366 |
| Non US | 7 (41) | 17 | 3 (20) | 15 | 1 (6) | 16 |
Source: Company Clinical Trial data
What are the possible side effects?
The most common side effects of VIBERZI are constipation, nausea and belly pain.
The most serious known risk is too much contraction of a small muscle in the gut (called the sphincter of Oddi) that controls the flow of juices into the small intestine from the liver and pancreas to help with food digestion. Contraction of this muscle can cause sudden pain in the belly and lead to abnormal results from liver and pancreas tests. These abnormal results may indicate damage to the liver and swelling of the pancreas (called pancreatitis).
Because of these side effects, VIBERZI should not be used in patients who have or have had problems with their sphincter of Oddi, pancreatitis, severe liver problems, severe constipation, or possible blockage of their gut. VIBERZI should also not be used in patients who drink more than three alcoholic beverages per day.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions for the Safety population, which includes any patient who received at least one dose of trial drug. The Safety population is a pooled population of two Phase 3 trials and one additional Phase 2 trial.
Table 5. Common* Adverse Reactions in the Pooled Trials (Safety population)
| Adverse Reactions | VIBERZI 100 mg (n= 1032) % |
VIBERZI 75 mg (n=807) % |
Placebo (n=975) % |
|---|---|---|---|
| Constipation | 8 | 7 | 3 |
| Nausea | 7 | 8 | 5 |
| Abdominal Pain** | 7 | 6 | 4 |
| Upper Respiratory Tract Infection | 5 | 3 | 4 |
| Vomiting | 4 | 4 | 1 |
| Nasopharyngitis | 3 | 4 | 3 |
| Abdominal Distention | 3 | 3 | 2 |
| Bronchitis | 3 | 3 | 2 |
| Dizziness | 3 | 3 | 2 |
| Flatulence | 3 | 3 | 2 |
| Rash*** | 3 | 3 | 2 |
| Increased ALT | 3 | 2 | 1 |
| Fatigue | 2 | 3 | 2 |
| Viral gastroenteritis | 1 | 3 | 2 |
*Reported in > 2% of VIBERZI-treated patients at either dose and at an incidence greater than in placebo-treated patients
**"Abdominal Pain" term includes: abdominal pain, abdominal pain lower, and abdominal pain upper*** "Rash" term includes: dermatitis, dermatitis allergic, rash, rash erythematous, rash generalized, rash maculo-papular, rash papular, rash pruritic, urticaria, and idiopathic urticaria
Source: VIBERZI Prescribing Information, Section 14, Table 1
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race, and age.
- Sex: The majority of patients in the trials were women. The risk of overall side effects was similar in men and women.
- Race: The majority of patients in the trials were white. Differences in side effects among races could not be determined.
- Age: Overall side effects were seen more often in patients 65 years of age and above compared to those below the age of 65. Side effects in the gut and certain side effects—called serious adverse events1 --were also seen more often in patients age 65 and above compared to those below the age of 65.
1Serious adverse event was defined as any event that resulted in one of the following: death, life-threatening event, required hospitalization or extended a current hospital stay, persistent or significant disability/incapacity, or congenital anomaly or birth defect
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The Prescribing Information for VIBERZI (Section 8.5) states that a higher proportion of elderly patients than younger patients experienced adverse reactions, serious adverse reactions, and gastrointestinal adverse reaction.
The table below summarizes adverse events in the pooled trials (Safety population) by subgroups.
Table 6. Subgroup Analysis of all Adverse Events While on Treatment (Safety Population)
| Subgroup | VIBERZI 100 mg | VIBERZI 75 mg | Placebo | |||
|---|---|---|---|---|---|---|
| x (%) | Total, n | x (%) | Total, n | x (%) | Total, n | |
| Any TEAEs* | 575 (56) | 1032 | 486 (60) | 807 | 533 (55) | 975 |
| Sex | ||||||
| Male | 177 (52) | 343 | 139 (52) | 270 | 156 (47) | 333 |
| Female | 398 (58) | 689 | 347 (65) | 537 | 377 (59) | 642 |
| Age Group | ||||||
| <65> | 526 (55) | 957 | 443 (60) | 742 | 465 (53) | 872 |
| >=65 years | 49 (65) | 75 | 43 (66) | 65 | 68 (66) | 103 |
| Race | ||||||
| White | 502 (57) | 875 | 427 (61) | 698 | 481 (57) | 847 |
| Black or African American | 57 (45) | 126 | 46 (50) | 92 | 40 (38) | 105 |
| Other | 16 (52) | 31 | 13 (77) | 17 | 12 (52) | 23 |
*TEAEs=treatment-emergent adverse events
Source: Company Clinical Trial Data
The table below summarizes gastrointestinal adverse events in the pooled trials (Safety population) by subgroups.
Table 7. Subgroup Analysis of Gastrointestinal AEs while on Treatment (Safety Population)
| Subgroup | VIBERZI 100 mg | VIBERZI 75 mg | Placebo | |||
|---|---|---|---|---|---|---|
| x (%) | Total, n | x (%) | Total, n | x (%) | Total, n | |
| Any GI AEs* | 273 (27) | 1032 | 242 (30) | 807 | 185 (19) | 975 |
| Sex | ||||||
| Male | 78 (23) | 343 | 61 (23) | 270 | 52 (16) | 333 |
| Female | 195 (28) | 689 | 181 (34) | 537 | 133 (21) | 642 |
| Age Group | ||||||
| <65> | 241 (25) | 957 | 220 (30) | 742 | 156 (18) | 872 |
| >=65 years | 32 (43) | 75 | 22 (34) | 65 | 29 (28) | 103 |
| Race | ||||||
| White | 237 (27) | 875 | 219 (31) | 698 | 165 (20) | 847 |
| Black or African American | 24 (19) | 126 | 17 (19) | 92 | 15 (14) | 105 |
| Other | 12 (39) | 31 | 6 (35) | 17 | 5 (22) | 23 |
*GI AEs= Gastrointestinal adverse events
Source: Company Clinical Trial Data
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved VIBERZI based on evidence from two clinical trials of 2425 patients with IBS-D. The trials were conducted at 556 sites in the United States, Canada, and United Kingdom.
Figure 1 summarizes how many men and women were enrolled in the clinical trials used to evaluate the benefit of VIBERZI.
Figure 1. Baseline Demographics by Sex
Source: Company Clinical Trial Data
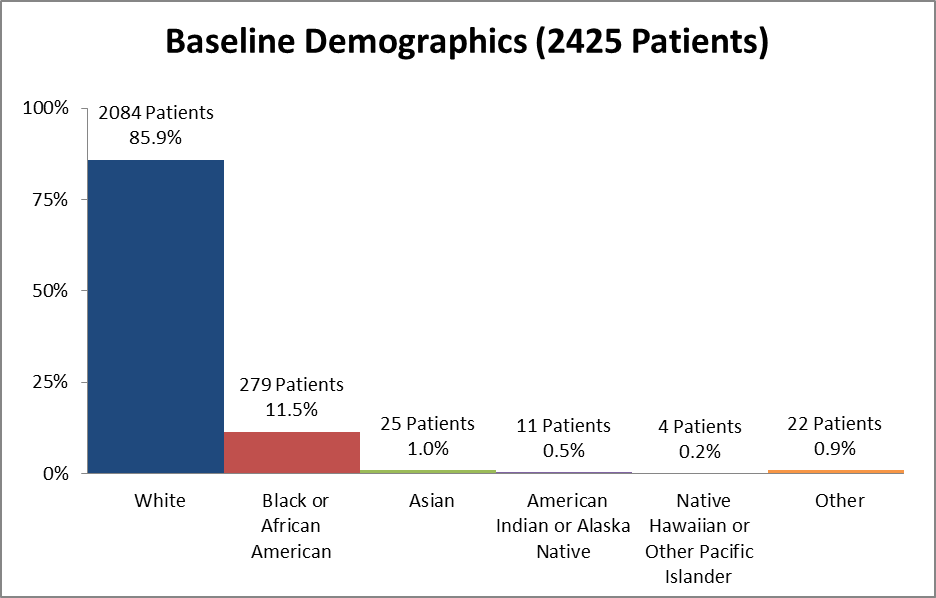
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trials used to evaluate benefit.
Figure 2. Baseline Demographics by Race
Source: Company Clinical Trial Data
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 2084 | 85.9% |
| Black or African American | 279 | 11.5% |
| Asian | 25 | 1.0% |
| American Indian or Alaska Native | 11 | 0.5% |
| Native Hawaiian or Other Pacific Islander | 4 | 0.2% |
| Other | 22 | 0.9% |
Source: Company Clinical Trial Data
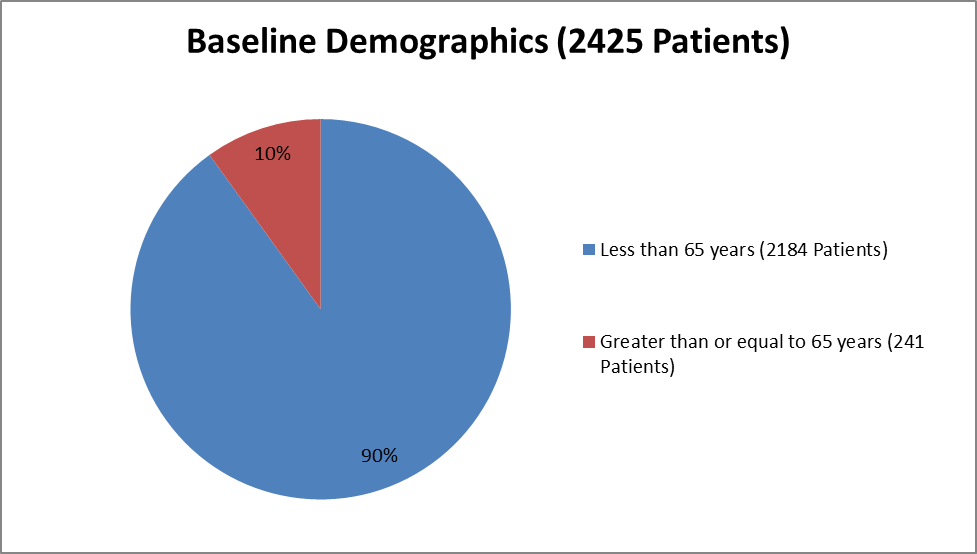
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trials used to evaluate benefit.
Figure 3. Baseline Demographics by Age
Source: Company Clinical Trial Data
Who participated in the trials?
The tables below summarize baseline demographics for the efficacy (ITT) and safety populations.
Table 8. Demographic and Baseline Characteristics (ITT Population)
| Demographic Parameters | Trial 1 | Trial 2 | Total (N=2425) n (%) |
||||
|---|---|---|---|---|---|---|---|
| VIBERZI 100 mg (N=426) n (%) |
VIBERZI 75 mg (N=427) n (%) |
Placebo (N=427) n (%) |
VIBERZI 100 mg (N=382) n (%) |
VIBERZI 75 mg (N=381) n (%) |
Placebo (N=382) n (%) |
||
| Sex | |||||||
| Male | 143 (34) | 151 (35) | 150 (35) | 126 (33) | 120 (32) | 132 (35) | 822 (34) |
| Female | 283 (66) | 276 (65) | 277 (65) | 256 (67) | 261 (68) | 250 (65) | 1603 (66) |
| Age | |||||||
| Mean years (SD) | 44.4 (13.91) | 44.5 (13.20) | 45.8 (14.10) | 45.7 (13.32) | 45.0 (13.17) | 47.1 (13.82) | 45.4 (13.62) |
| Median (years) | 45.0 | 44.0 | 45.0 | 45.0 | 45.0 | 47.5 | 45.0 |
| Min, Max (years) | 18, 79 | 18, 80 | 18, 79 | 19, 75 | 18, 77 | 19, 77 | 18, 80 |
| Age Group | |||||||
| <65> | 391 (92) | 398 (93) | 376 (88) | 343 (90) | 345 (91) | 331 (87) | 2184 (90) |
| >=65 years | 35 (8) | 29 (7) | 51 (12) | 39 (10) | 36 (9) | 51 (13) | 241 (10) |
| Race | |||||||
| White | 368 (86) | 372 (87) | 370 (87) | 318 (83) | 327 (86) | 329 (86) | 2084 (86) |
| Black or African American | 48 (11) | 46 (11) | 46 (11) | 50 (13) | 46 (12) | 43 (11) | 279 (12) |
| Asian | 3 (1) | 3 (1) | 4 (1) | 7 (2) | 2 (<> | 6 (2) | 25 (1) |
| American Indian or Alaska Native | 2 (1) | 1 (<> | 1 (<> | 3 (1) | 3 (1) | 1 (<> | 11 (<> |
| Native Hawaiian or Other Pacific Islander | 1 (<> | 0 | 0 | 1 (<> | 0 | 2 (1) | 4 (<> |
| Other | 4 (1) | 5 (1) | 6 (1) | 3 (1) | 3 (1) | 1 (<> | 22 (1) |
| Ethnicity | |||||||
| Hispanic or Latino | 117 (28) | 119 (28) | 125 (29) | 99 (26) | 98 (26) | 101 (26) | 659 (27) |
| Not Hispanic or Latino | 309 (72) | 308 (72) | 302 (71) | 283 (74) | 283 (74) | 281 (74) | 1766 (73) |
| Region | |||||||
| United States | 404 (95) | 403 (94) | 405 (95) | 365 (96) | 366 (96) | 366 (96) | 2309 (95) |
| Non US | 22 (5) | 24 (6) | 22 (5) | 17 (4) | 15 (4) | 16 (4) | 116 (5) |
Source: Company Clinical Trial Data
Table 9. Demographic and Baseline Characteristics (Safety Population)
| Demographic Parameters | VIBERZI 100 mg (N=1032) n (%) |
VIBERZI 75mg (N=807) n (%) |
Placebo (N=975) n (%) |
Total (N=2814) n (%) |
|---|---|---|---|---|
| Sex | ||||
| Male | 343 (33) | 270 (34) | 333 (34) | 946 (34) |
| Female | 689 (67) | 537 (66) | 642 (66) | 1868 (66) |
| Age | ||||
| Mean years (SD) | 44.8 (13.13) | 44.8 (13.17) | 46.0 (13.72) | 45.22 (13.36) |
| Median (years) | 45.0 | 44.0 | 46.0 | 45.0 |
| Min, Max (years) | 18, 79 | 18, 80 | 18, 79 | 18, 80 |
| Age Group | ||||
| <65> | 957 (93) | 742 (93) | 872 (89) | 2571 (91) |
| >=65 years | 75 (7) | 65 (8) | 103 (11) | 243 (9) |
| Race | ||||
| White | 875 (85) | 698 (87) | 847 (87) | 2420 (86) |
| Black or African American |
126 (12) | 92 (11) | 105 (11) | 323 (11) |
| Asian | 13 (1) | 5 (1) | 12 (1) | 30 (1) |
| American Indian or Alaska Native |
5 (1) | 4 (<> | 2 (<> | 11 (<> |
| Native Hawaiian or Other Pacific Islander |
2 (<> | 0 | 2 (<> | 4 (<> |
| Other | 11 (1) | 8 (1) | 7 (1) | 26 (1) |
| Ethnicity | ||||
| Hispanic or Latino | 261 (25) | 214 (27) | 251 (26) | 726 (26) |
| Not Hispanic or Latino | 771 (71) | 593 (73) | 724 (74) | 2088 (74) |
| Region | ||||
| United States | 991 (96) | 768 (95) | 937 (96) | 2696 (96) |
| Non US | 41 (4) | 39 (5) | 38 (4) | 118 (4) |
Source: Company Clinical Trial Data
How were the trials designed?
There were two trials that evaluated the benefit and side effects of VIBERZI. In each trial, patients were randomly assigned to receive either VIBERZI 100 mg or VIBERZI 75 mg or placebo two times daily for at least 26 weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
The trials measured improvements at 12 weeks in abdominal pain and watery stool and compared VIBERZI to placebo.
How were the trials designed?
Two randomized, multi-center, double-blind, placebo-controlled trials were conducted to assess the efficacy and safety of 12 weeks of twice daily treatment with VIBERZI 100 mg or VIBERZI 75 mg in comparison to placebo in patients with irritable bowel syndrome with diarrhea.
The efficacy was assessed using a composite endpoint which included improvement in the daily worst abdominal pain score by ≥30% as compared to the baseline weekly average AND a reduction in the Bristol Stool Scale to <5 on="" at="" least="" 50%="" of="" the="" days="" within="" a="" 12-week="" time="">
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.