Drug Trials Snapshots: VEKLURY
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the VEKLURY Package Insert for complete information.
VEKLURY (remdesivir)
VEK-lur-ee
Gilead Sciences, Inc.
Approval date: October 22, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VEKLURY is for use in adult and pediatric patients 12 years and older weighing at least 40 kg (88 lbs) for the treatment of coronavirus disease 2019 (COVID-19) requiring hospitalization.
COVID-19 is an infectious, potentially serious or life-threating respiratory disease caused by a coronavirus called SARS-CoV-2 virus.
How is this drug used?
VEKLURY is an injection. It is given by a healthcare provider directly into the vein (intravenous infusion) once a day for 5 to 10 days.
What are the benefits of this drug?
In one trial in patients with mild, moderate and severe COVID-19, the time to recovery was shorter in patients who received VEKLURY than in patients who received placebo. In a different trial in patients with moderate COVID-19, treatment with VEKLURY was better in improving patients’ condition in comparison to standard of care.
What are the benefits of this drug (results of trials used to assess efficacy)?
The results of primary endpoint efficacy analyses for individual trials are presented below.
Trial 1
The primary clinical endpoint was time to recovery within 29 days after randomization. The median time to recovery was 10 days in the VEKLURY group compared to 15 days in the placebo group (recovery rate ratio 1.29 [95% CI 1.12 to 1.49], p<0.001). Among patients with mild/moderate disease at enrollment (n=105), the median time to recovery was 5 days in both the VEKLURY and placebo groups (recovery rate ratio 1.22 [95% CI 0.82 to 1.81]). Among patients with severe disease at enrollment (n=957), the median time to recovery was 11 days in the VEKLURY group compared to 18 days in the placebo group (recovery rate ratio 1.31 [95% CI 1.12 to 1.52]).
Trial 2
The primary endpoint was clinical status on Day 14 assessed on a 7-point ordinal scale. Overall, after adjusting for between-group differences at baseline, patients receiving a 5-day course of VEKLURY had similar clinical status at Day 14 as those receiving a 10-day course (odds ratio for improvement: 0.75; [95% CI 0.51 to 1.12]).
Trial 3
The primary endpoint was clinical status on Day 11 assessed on a 7-point ordinal scale Overall, the odds of improvement in the ordinal scale were higher in the 5-day VEKLURY group at Day 11 when compared to those receiving only standard of care (odds ratio, 1.65; [95% CI, 1.09 to 2.48], p=0.017). The odds of improvement in clinical status with the 10-day treatment group when compared to those receiving only standard of care were not statistically significant (odds ratio 1.31; [95% CI 0.88 to 1.95]).
VEKLURY Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: VEKLURY worked similarly in men and women.
- Race: VELKURY worked similarly in all tested race groups.
- Age: VEKLURY worked similarly in all tested age groups including patients 65 years and older.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize primary efficacy results by sex, race and age subgroups for individual trials.
Table 1. Subgroup Analysis: Time to Recovery-Trial 1
| Demographic Category | Number of Patients | Recovery Rate Ratio (95% CI) |
|---|---|---|
| Sex | ||
| Men | 684 | 1.31 (1.1 to 1.57) |
| Women | 378 | 1.32 (1.04 to 1.67) |
| Race | ||
| White | 566 | 1.3 (1.07 to 1.59) |
| Black or African American | 226 | 1.26 (0.92 to 1.73) |
| Asian | 135 | 1.08 (0.73 to 1.59) |
| Age | ||
| <40 years | 119 | 2 (1.31 to 3.03) |
| 40-64 years | 559 | 1.2 (0.99 to 1.45) |
| ≥65 years | 384 | 1.3 (1 to 1.68) |
CI=confidence interval
Adapted from FDA Statistical Review
Table 2. Subgroup Analysis: Day 14 Ordinal Scale Results-Trial 2
| Demographic Category | Number of Patients | Odds Ratio for Day 14 (95% CI) |
|---|---|---|
| Sex | ||
| Men | 253 | 0.81 (0.51 to 1.28) |
| Women | 144 | 0.5 (0.26 to 0.97) |
| Race | ||
| White | 276 | 0.62 (0.4 to 0.98) |
| Black or African American | 44 | 1.47 (0.39 to 5.52) |
| Asian | 45 | 0.78 (0.26 to 2.34) |
| Age | ||
| <40 years | 41 | 1.14 (0.22 to 5.86) |
| 40-64 years | 188 | 0.97 (0.54 to 1.72) |
| ≥65 years | 168 | 0.45 (0.26 to 0.79) |
CI=confidence interval
Adapted from FDA Statistical Review
Table 3. Subgroup Analysis: Day 11 Ordinal Scale Results -Trial 3
| Demographic Category | Number of Patients | Odds Ratio for VEKLURY 5 days versus SOC (95% CI) | Odds Ratio for VEKLURY 10 days versus SOC (95% CI) |
|---|---|---|---|
| Sex | |||
| Men | 357 | 1.59 (0.95 to 2.66) |
1.45 (0.88 to 2.41) |
| Women | 227 | 1.73 (0.87 to 3.41) |
1.11 (0.58 to 2.12) |
| Race | |||
| White | 328 | 1.51 (0.88 to 2.58) |
1.61 (0.93 to 2.78) |
| Black or African American | 99 | 7.81 (0.85 to 71.4) |
0.96 (0.27 to 3.42) |
| Asian | 102 | 1.62 (0.66 to 3.93) |
0.61 (0.24 to 1.55) |
| Age | |||
| <40 years | 92 | 2.09 (0.69 to 6.39) |
1.68 (0.61 to 4.69) |
| 40-64 years | 333 | 1.58 (0.87 to 2.84) |
1.03 (0.59 to 1.81) |
| ≥65 years | 159 | 1.65 (0.81 to 3.37) |
1.59 (0.78 to 3.22) |
SOC=Standard of care; CI=confidence interval
Adapted from FDA Statistical Review
What are the possible side effects?
VEKLURY may cause serious side effects including allergic reactions during and after the infusion, and increased liver enzymes in the blood.
The most common side effects of VEKLURY are nausea and increased liver enzymes in the blood.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions from each trial separately.
Table 4. Summary of Adverse Reaction Rates in Patients with Mild, Moderate or Severe COVID-19 in Trial 1
| Types of Adverse Reactions | VEKLURY (N=532) n (%) |
Placebo (N=516) n (%) |
|---|---|---|
| Adverse Reactions, Grades ≥3 | 41 (8%) | 46 (9%) |
| Serious adverse reactions | 2 (0.4%)a | 3 (0.6%) |
| Adverse reactions leading to treatment discontinuation | 11 (2%)b | 15 (3%) |
a. Seizure (n=1), infusion-related reaction (n=1).
b. Seizure (n=1), infusion-related reaction (n=1), transaminases increased (n=3), ALT increased and AST increased (n=1), GFR decreased (n=2), acute kidney injury (n=3).
Table 5. Summary of Adverse Reaction Rates in Patients with Severe COVID-19 in Trial 2
| Types of Adverse Reactions | VEKLURY 5 Days (N=200) |
VEKLURY 10 Days (N=197) |
|---|---|---|
| Any adverse reaction, all Grades | 33 (17%) | 40 (20%) |
| Serious adverse reactions | 3 (2%)a | 4 (2%)a |
| Adverse reactions leading to treatment discontinuationc | 5 (3%)b | 9 (5%)b |
a. Transaminases increased (n=5), hepatic enzyme increased (n=1), hypertransaminasaemia (n=1).
b. Transaminases increased (n=4), hepatic enzyme increased (n=2), LFT increased (n=2), hypertransaminasaemia (n=1), ALT increased (n=1), ALT increased and AST increased (n=2), injection site erythema (n=1), rash (n=1).
Table 6. Summary of Adverse Reactiona Rates in Patients with Moderate COVID-19 in Trial 3
| Types of Adverse Reactions | VEKLURY 5 Days (N=191) |
VEKLURY 10 Days (N=193) |
|---|---|---|
| Any adverse reaction, all Grades | 36 (19%) | 25 (13%) |
| Serious adverse reactions | 1 (<1%)b | 0 |
| Adverse reactions leading to treatment discontinuationc | 4 (2%)c | 4 (2%)c |
a. Attribution of events to study drug was not performed for the SOC group.
b. Heart rate decreased.
c. ALT increased (n=2), ALT increased and AST increased (n=1), hypertransaminasaemia (n=1), blood alkaline phosphatase increased (n=1), rash (n=2), heart rate decreased (n=1).
VEKLURY Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in all tested race groups.
- Age: The occurrence of side effects was lower in patients younger than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the most frequent adverse events (AEs) by sex, race and age subgroups.
Table 7a. Subgroup Analysis of Adverse Events by Sex -Trial 1
| VEKLURY N=532 |
Placebo N=516 |
|||
|---|---|---|---|---|
| Women N=185 n (%) |
Men N=347 n (%) |
Women N=188 n (%) |
Men N=328 n (%) |
|
| Any AE | 105 (57) | 200 (58) | 120 (64) | 203 (62) |
| Any Serious AE | 44 (24) | 87 (25) | 51 (27) | 112 (34) |
| AE leading to discontinuation of study drug | 18 (10) | 39 (11) | 28 (15) | 49 (15) |
Table 7b. Subgroup Analysis of Adverse Events by Sex -Trial 2
| VEKLURY 5 Days N=200 |
VEKLURY 10 Days N=197 |
|||
|---|---|---|---|---|
| Women N=80 n (%) |
Men N=120 n (%) |
Women N=64 n (%) |
Men N=133 n (%) |
|
|
Any AE |
57 (71) | 86 (72) | 45 (70) | 103 (77) |
|
Any Serious AE |
8 (10) | 35 (29) | 16 (25) | 52 (39) |
|
AE leading to discontinuation of study drug |
0 | 9 (8) | 4 (6) | 18 (13) |
Table 7c. Subgroup Analysis of Adverse Events by Sex -Trial 3
| VEKLURY 5 Days + SOC N=191 |
VEKLURY 10 Days + SOC N=193 |
SOC N=200 |
||||
|---|---|---|---|---|---|---|
| Women N=77 n (%) |
Men N=114 n (%) |
Women N=75 n (%) |
Men N=118 n (%) |
Women N=75 n (%) |
Men N=125 n (%) |
|
| Any AE | 46 (60) | 52 (46) | 45 (60) | 68 (58) | 37 (4) | 56 (45) |
| Any Serious AE | 2 (3) | 7 (6) | 3 (4) | 7 (6) | 4 (5) | 14 (11) |
| AE leading to discontinuation of study drug | 1 (1) | 3 (3) | 1 (1) | 7 (6) | 0 | 0 |
SOC=standard of care
Table 8a. Subgroup Analysis of Adverse Events by Race -Trial 1
| VEKLURY N=532 |
Placebo N=516 |
|||||||
|---|---|---|---|---|---|---|---|---|
| White N=273 n (%) |
Black N=105 n (%) |
Asian N=79 n (%) |
Other N=75 n (%) |
White N=286 n (%) |
Black N=114 n (%) |
Asian N=56 n (%) |
Other N=60 n (%) |
|
| Any AE | 151 (55.3) | 70 (66.7) | 45 (57) | 39 (52) | 172 (60.1) | 80 (70.2) | 25 (44.6) | 46 (76.7) |
| Any Serious AE | 64 (23.4) | 34 (32.4) | 19 (24.1) | 14 (18.7) | 87 (30.4) | 40 (35.1) | 13 (23.2) | 23 (38.3) |
| AE leading to discontinuation of study drug | 26 (9.5) | 18 (17.1) | 8 (10.1) | 5 (6.7) | 39 (13.6) | 23 (20.2) | 6 (10.7) | 9 (15) |
Table 8b. Subgroup Analysis of Adverse Events by Race-Trial 2
| VEKLURY 5 Days N=200 |
VEKLURY 10 Days N=197 |
|||||||
|---|---|---|---|---|---|---|---|---|
| White N=142 n (%) |
Black N=21 n (%) |
Asian N=20 n (%) |
Other N=17 n (%) |
White N=134 n (%) |
Black N=23 n (%) |
Asian N=25 n (%) |
Other N=15 n (%) |
|
| Any AE | 102 (72) | 14 (67) | 15 (75) | 12 (71) | 102 (76) | 14 (61) | 21 (84) | 11 (73) |
| Any Serious AE | 29 (20) | 2 (10) | 8 (40) | 4 (24) | 52 (39) | 3 (13) | 6 (24) | 7 (47) |
| AE leading to discontinuation of study drug | 8 (6) | 0 | 1 (5) | 0 | 13 (10) | 4 (17) | 3 (12) | 2 (13) |
Table 8c. Subgroup Analysis of Adverse Events by Race-Trial 3
| VEKLURY 5 Days + SOC N=191 |
VEKLURY 10 Days + SOC N=193 |
SOC N=200 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White N=109 n (%) |
Black N=35 n (%) |
Asian N=34 n (%) |
Other N=13 n (%) |
White N=107 n (%) |
Black N=37 n (%) |
Asian N=31 n (%) |
Other N=18 n (%) |
White N=112 n (%) |
Black N=27 n (%) |
Asian N=37 n (%) |
Other N=24 n (%) |
|||
| Any AE | 60 (55) | 14 (40) | 19 (56) | 5 (39) | 66 (62) | 19 (51) | 18 (58) | 10 (6) | 58 (52) | 8 (30) | 15 (41) | 12 (50) | ||
| Any Serious AE | 5 (5) | 3 (9) | 1 (3) | 0 | 4 (4) | 4 (11) | 0 | 2 (11) | 10 (9) | 5 (19) | 1 (3) | 2 (8) | ||
| AE leading to discontinuation of study drug | 1 (1) | 0 | 2 (6) | 1 (8) | 3 (3) | 3 (8) | 0 | 2 (11) | 0 | 0 | 0 | 0 | ||
SOC=standard of care
Table 9a. Subgroup Analysis of Adverse Events by Age-Trial 1
| VEKLURY N=532 |
Placebo N=516 |
|||
|---|---|---|---|---|
| Age < 65 years N=349 n (%) |
Age >= 65 years N=183 n (%) |
Age < 65 years N=321 n (%) |
Age >= 65 years N=195 n (%) |
|
| Any Adverse Event | 185 (53) | 120 (66) | 187 (58) | 136 (70) |
| Any Serious Adverse Event | 68 (20) | 63 (34) | 88 (27) | 75 (39) |
| Adverse Event leading to discontinuation of study drug | 32 (9) | 25 (14) | 44 (14) | 33 (17) |
Table 9b. Subgroup Analysis of Adverse Events by Age-Trial 2
| VEKLURY 5 Days N=200 |
VEKLURY 10 Days N=197 |
|||
|---|---|---|---|---|
| Age < 65 years N=116 n (%) |
Age >= 65 years N=84 n (%) |
Age < 65 years N=113 n (%) |
Age >= 65 years N=84 n (%) |
|
| Any AE | 80 (69) | 63 (75) | 74 (66) | 74 (88) |
| Any Serious AE | 22 (19) | 21 (25) | 24 (21) | 44 (52) |
| AE leading to discontinuation of study drug | 6 (5) | 3 (4) | 12 (11) | 10 (12) |
Table 9c. Subgroup Analysis of Adverse Events by Age-Trial 3
| VEKLURY 5 Days + SOC N=191 |
VEKLURY 10 Days + SOC N=193 |
SOC N=200 |
||||
|---|---|---|---|---|---|---|
| Age < 65 years N=142 n (%) |
Age >= 65 years N=49 n (%) |
Age < 65 years N=141 n (%) |
Age >= 65 years N=52 n (%) |
Age < 65 years N=142 n (%) |
Age >= 65 years N=58 n (%) |
|
| Any AE | 69 (49) | 29 (59) | 80 (57) | 33 (64) | 58 (41) | 35 (60) |
| Any Serious AE | 4 (3) | 5 (10) | 5 (4) | 5 (10) | 7 (5) | 11 (19) |
| AE leading to discontinuation of study drug | 4 (3) | 0 | 8 (6) | 0 | 0 | 0 |
SOC=Standard of care
Clinical Trials Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved VEKLURY based primarily on evidence from three clinical trials (Trial 1/ NCT NCT04280705, Trial 2/ NCT04292899) and Trial 3/ NCT04292730) of 2043 hospitalized patients with COVID-19. The trials were conducted at 226 sites in 17 countries including the United States.
The figures below present combined patients from all three trials that provided data for the assessment of VEKLURY benefits (efficacy population).
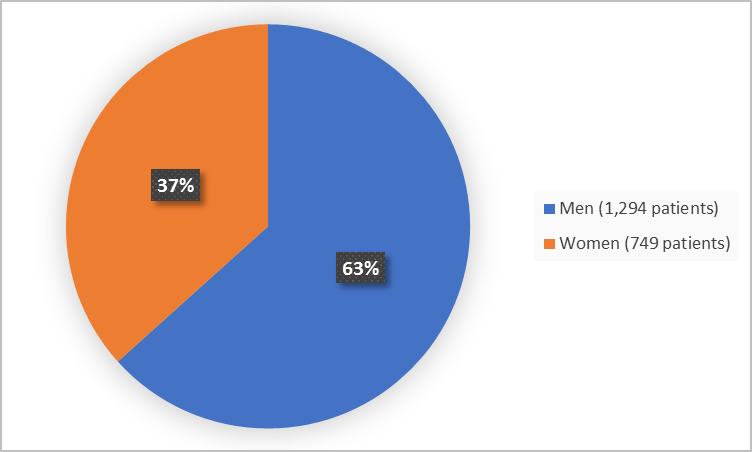
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Demographics by Sex (efficacy population)
FDA Clinical Review
Figure 2 summarizes the percentage of patients by race.
Figure 2. Demographics by Race (efficacy population)
*Includes Multiple and Other
FDA Clinical Review
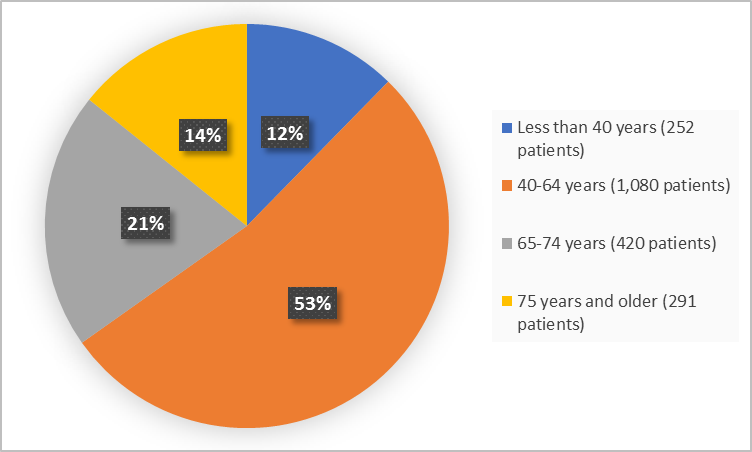
Figure 3 summarizes the percentage of patients by age.
Figure 3. Demographics by Age (efficacy population)
FDA Clinical Review
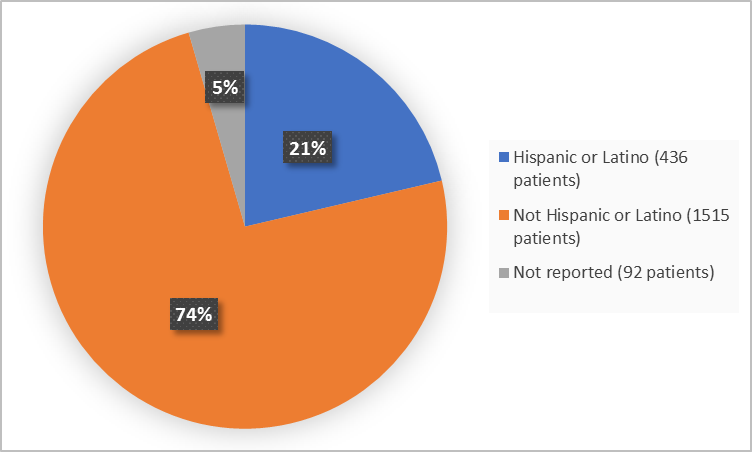
Figure 4 summarizes the percentage of patients by ethnicity.
Figure 4. Demographics by Ethnicity (efficacy population)
FDA Clinical Review
Who participated in the trials?
The table below summarizes demographics of patients in the trial.
Table 10. Demographic Characteristics (ITT population)
| Demographic Parameters | Trial 1 (N=1062) n (%) |
Trial 2 (N=397) n (%) |
Trial 3 N=584 n (%) |
TOTAL N=2043 n (%) |
|---|---|---|---|---|
| Sex | ||||
| Men | 684 (64%) | 253 (64%) | 357 (61%) | 1,294 (63%) |
| Women | 378 (36%) | 144 (36%) | 227 (39%) | 749 (37%) |
| Race | ||||
| White | 566 (53%) | 276 (70%) | 328 (56%) | 1,170 (57%) |
| Black or African American | 225 (21%) | 44 (11%) | 99 (17%) | 368 (18%) |
| Asian | 135 (13%) | 45 (11%) | 102 (17.5%) | 282 (14%) |
| American Indian or Alaska Native | 7 (1%) | 2 (1%) | 3 (0.5%) | 12 (1%) |
| Native Hawaiian or Other Pacific Islander | 4 (<1%) | 1 (<1) | 3 (0.5%) | 8 (<1%) |
| Other1 | 125 (12%) | 24 (6%) | 32 (5.5%) | 181 (9%) |
| Not Reported | 0 | 5 (1) | 17 (3%) | 22 (1%) |
| Age | ||||
| Median (years) | 59 | 58 | 57 | 59 |
| Min, max (years) | 21, 95 | 24, 85 | 23, 95 | 21, 95 |
| Age Group | ||||
| <40 years | 119 (11%) | 41 (10%) | 92 (16%) | 252 (12%) |
| 40 - 64 years | 559 (53%) | 188 (47%) | 333 (57%) | 1,080 (53%) |
| 65-74 years | 220 (21%) | 105 (26%) | 95 (16%) | 420 (21%) |
| ≥ 75 years | 164 (15%) | 63 (16%) | 64 (11%) | 291 (14%) |
| Ethnicity | ||||
| Hispanic or Latino | 250 (25%) | 85 (21%) | 101 (17%) | 436 (21%) |
| Not Hispanic or Latino | 755 (71%) | 302 (76%) | 458 (78%) | 1515 (74%) |
| Not reported | 57 (5%) | 10 (3%) | 25 (4%) | 92 (5%) |
| Region | ||||
| United States | 837 (79%) | 229 (58%) | 260 (44.5%) | 1,326 (65%) |
| Denmark | 43 (4%) | 0 | 0 | 43 (2%) |
| Spain | 28 (3%) | 61 (15%) | 96 (16%) | 185 (9%) |
| Italy | 0 | 77 (19%) | 79 (14%) | 156 (8%) |
| United Kingdom | 46 (4%) | 0 | 33 (6%) | 79 (4%) |
| South Korea | 21 (2%) | 12 (3%) | 21 (3%) | 54 (3%) |
| Singapore | 16 (2%) | 9 (2%) | 18 (3%) | 43 (2%) |
| Germany | 13 (1%) | 4 (1%) | 22 (4%) | 39 (2%) |
| Hong Kong | 0 | 4 (1%) | 27 (4%) | 31 (1.5%) |
| Greece | 22 (2%) | 0 | 0 | 22 (1%) |
| Japan | 15 (1%) | 0 | 0 | 15 (1%) |
| Switzerland | 0 | 0 | 15 (3%) | 15 (1%) |
| North Macedonia | 11 (1%) | 0 | 0 | 11 (0.5%) |
| Mexico | 10 (1%) | 0 | 0 | 10 (0.5%) |
| Taiwan | 0 | 1 (<0.5%) | 6 (1%) | 7 (<1%) |
| Netherlands | 0 | 0 | 4 (1%) | 4 (<1%) |
| France | 0 | 0 | 3 (0.5%) | 3 (<1%) |
Adapted from FDA Review
How were the trials designed?
There were three trials that evaluated benefits and side effects of VEKLURY. Each trial was designed differently.
Trial 1 enrolled hospitalized adult patients with mild, moderate or severe COVID-19. Patients received at random either VEKLURY or placebo infusion every day for 10 days. Neither the patients nor the investigators knew which treatment was given. The benefit was evaluated by comparing the time to recovery within 29 days in VEKLURY-treated group to the placebo-treated group.
Trial 2 enrolled hospitalized adult patients with severe COVID-19. All patients had pneumonia. Patients received at random VEKLURY treatment once a day for either 5 or 10 days. The benefit was evaluated after 14 days of treatment by comparing the patients’ condition between the two groups. The patient’s condition was assessed on a 7-point scale going from “death” to “not hospitalized”.
Trial 3 enrolled hospitalized adult patients with moderate COVID-19. All patients had pneumonia. Patients received at random VEKLURY treatment once a day for either 5 or 10 days (in addition to standard of care), or the standard of care only. The benefit was evaluated after 11 days of treatment by comparing the patients’ condition among the three groups. The patient’s condition was assessed using the same scale as in Trial 2.
How were the trials designed?
The safety and efficacy of VEKLURY were established in three clinical trials.
Trial 1 was a randomized, double-blind, placebo-controlled clinical trial of hospitalized adult patients with confirmed SARS-CoV-2 infection and mild, moderate, or severe COVID-19 which compared treatment with VEKLURY for 10 days with placebo. The primary clinical endpoint was time to recovery within 29 days after randomization. Recovery was defined as discharged from the hospital without limitations on activities, discharged from the hospital with limitations on activities and/or requiring home oxygen, or hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care.
Trial 2 was a randomized, open-label, multi-center clinical trial in hospitalized adult patients with confirmed SARS-CoV-2 infection, an SpO2 of ≤94% on room air, and radiological evidence of pneumonia which compared 5 days of VEKLURY treatment with 10 days of VEKLURY treatment. The primary endpoint was clinical status on Day 14 assessed on a 7-point ordinal scale consisting of the following categories:
- death;
- hospitalized, receiving invasive mechanical ventilation or ECMO;
- hospitalized, receiving noninvasive ventilation or high-flow oxygen devices;
- hospitalized, requiring low-flow supplemental oxygen;
- hospitalized, not requiring supplemental oxygen but receiving ongoing medical care (related or not related to COVID-19);
- hospitalized, requiring neither supplemental oxygen nor ongoing medical care (other than that specified in the protocol for remdesivir administration); and
- not hospitalized.
Trial 3 was a randomized, open-label. multi-center clinical trial of hospitalized adult patients with confirmed SARS-CoV-2 infection, SpO2 >94% and radiological evidence of pneumonia. The trial compared treatment with VEKLURY for 5 days, treatment with VEKLURY for 10 days, and standard of care. The primary endpoint was clinical status on Day 11 assessed on a 7-point ordinal scale as described in Trial 2.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION