Drug Trials Snapshots: TALTZ
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to TALTZ Prescribing Information for complete information.
TALTZ (ixekizumab)
tȯl(t)s
Eli Lilly and Company
Approval date: March 22, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TALTZ is a drug for treatment of moderate to severe plaque psoriasis in adults.
How is this drug used?
TALTZ is an injection given once every other week over a period of twelve weeks followed by an injection once every four weeks.
What are the benefits of this drug?
TALTZ improved symptoms of plaque psoriasis and sustained the improvement through a year of treatment.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the clinical trials based on ITT (Intend to Treat Population).
Table 2. Efficacy Results at Week 12 in Adults with Plaque Psoriasis in Trials 1, 2, and 3; NRIa
| Trial 1 | Trial 2 | Trial 3 | ||||
|---|---|---|---|---|---|---|
| TALTZ (N=433) n (%) | Placebo (N=431) n (%) | TALTZ (N=351) n (%) | Placebo (N=168) n (%) | TALTZ (N=385) n (%) | Placebo (N=193) n (%) | |
| sPGA of “0” (clear) or “1” (minimal)b | 354 (82) | 14 (3) | 292 (83) | 4 (2) | 310 (81) | 13 (7) |
| sPGA of “0” (clear) | 160 (37) | 0 | 147 (42) | 1 (1) | 155 (40) | 0 |
| PASI 75b | 386 (89) | 17 (4) | 315 (90) | 4 (2) | 336 (87) | 14 (7) |
| PASI 90 | 307 (71) | 2 (1) | 248 (71) | 1 (1) | 262 (68) | 6 (3) |
| PASI 100 | 153 (35) | 0 | 142 (40) | 1 (1) | 145 (38) | 0 |
a Non-Responder Imputation
b Co-primary endpoints
sPGA= static Physician Global Assessment
PASI= Psoriasis Area and Severity Index
TALTZ Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: TALTZ worked similarly in men and women.
- Race: The majority of patients were white. The number of non-white patients were limited; therefore, differences in response among races could not be determined.
- Age: The number of patients above 65 years of age was limited; therefore, differences in response between patients above and below 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by subgroup for each trial (Trials 1, 2 and 3) based on ITT-NRI (Intend to Treat- Non-Responder Imputation) population.
Table 3. Results for the Co-Primary Efficacy Endpoints at Week 12 by Sex, Race, and Age, for Trial 1 (ITT, NRI)
| sPGA of 0 or 1 | PASI-75 | |||
|---|---|---|---|---|
| Subgroup (NTALTZ, NPlacebo) | TALTZ (N=433) | Placebo (N=431) | TALTZ (N=433) | Placebo (N=431) |
| Sex | ||||
| Race | ||||
| Age group | ||||
| Female (142,128) | 80% | 4% | 89% | 3% |
| Male (291,303) | 83% | 3% | 89% | 4% |
| Non-White (32, 30) | 78% | 3% | 84% | 7% |
| White (401, 401) | 82% | 3% | 90% | 4% |
| < 65="" (407,=""> | 82% | 3% | 89% | 4% |
| ≥ 65 (26,38) | 85% | 8% | 92% | 8% |
Adapted from FDA Statistical review
Table 4. Results for the Co-Primary Efficacy Endpoints at Week 12 by Sex, Race, and Age, for Trial 2 (ITT, NRI)
| sPGA of 0 or 1 | PASI-75 | |||
|---|---|---|---|---|
| Subgroup (NTALTZ, NPlacebo) | TALTZ (N=351) | Placebo (N=168) | TALTZ (N=351) | Placebo (N=168) |
| Sex | ||||
| Race | ||||
| Age group | ||||
| Female (130, 48) | 82% | 4% | 88% | 4% |
| Male (221, 120) | 84% | 2% | 91% | 2% |
| Non-White (21, 19) | 71% | 0% | 86% | 0% |
| White (330,149) | 84% | 3% | 90% | 3% |
| < 65="" (327,=""> | 83% | 3% | 89% | 3% |
| ≥ 65 (24, 9) | 88% | 0% | 96% | 0% |
Adapted from FDA Statistical review
Table 5. Results for the Co-Primary Efficacy Endpoints at Week 12 by Sex, Race, and Age, for Trial 3 (ITT, NRI)
| sPGA of 0 or 1 | PASI-75 | |||
|---|---|---|---|---|
| Subgroup (NTALTZ, NPlacebo) | TALTZ (N=385) | Placebo (N=193) | TALTZ (N=385) | Placebo (N=193) |
| Sex | ||||
| Race | ||||
| Age group | ||||
| Female (131, 56) | 80% | 7% | 89% | 11% |
| Male (254, 137) | 81% | 7% | 86% | 6% |
| Non-White (24, 17) | 67% | 0% | 88% | 12% |
| White (361, 176) | 81% | 7% | 87% | 7% |
| < 65="" (351,=""> | 81% | 7% | 87% | 8% |
| ≥ 65 (34, 13) | 79% | 0% | 94% | 0% |
Adapted from FDA Statistical review
What are the possible side effects?
The most common side effects of TALTZ are injection site reactions, upper respiratory tract infections, nausea, and fungal infections.
TALTZ may cause serious side effects including infections, allergic reactions and “flare-ups” of ulcerative colitis and Crohn’s disease.
Before starting TALTZ, patients should be evaluated for tuberculosis infection.
What are the possible side effects?
The table below summarizes adverse reactions that occurred in clinical trials.
Table 7. Adverse Reactions Occurring in ≥1% of the TALTZ Group and More Frequently than in the Placebo Group in the Plaque Psoriasis Clinical Trials through Week 12
| Adverse Reactions | TALTZ (N=1167) n(%) | Etanerceptb (N=287) n(%) | Placebo (N=791) n(%) |
| Injection site reactions | 196 (17) | 32 (11) | 26 (3) |
| Upper respiratory tract infectionsa | 163 (14) | 23 (8) | 101 (13) |
| Nausea | 23 (2) | 1 (<> | 5 (1) |
| Tinea infections | 17 (2) | 0 | 1 (<> |
a Upper respiratory tract infections cluster includes nasopharyngitis and rhinovirus infection.
b U.S. approved etanercept.
TALTZ Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex:The risk of side effects was similar in men and women.
- Race: The majority of patients were white. The number of non-white patients were limited; therefore, differences in side effects among races could not be determined.
- Age: The majority of patients were below 65 years of age. The difference between patients below and above age 65 years could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes treatment emergent adverse events (TEAEs) by subgroup for the pooled clinical trials.
Table 8. Subgroup Analysis of TEAEs
| Subgroup | TALTZ (N=1167) N/n(%) | Placebo (N=791) N/n(%) |
|---|---|---|
| Sex | ||
| Age Group | ||
| Race | ||
| Patients with ≥ 1 TEAE | 681/1167 (58) | 370/791 (47) |
| Male | 440/766 (57) | 248/559 (44) |
| Female | 241/401 (60) | 122/232 (53) |
| <65> | 638/1083 (59) | 173/731 (24) |
| ≥65 years | 43/84 (51) | 24/60 (40) |
| White | 638/1091 (56) | 341/725 (47) |
| Black or African American | 11/18 (61) | 9/26 (35) |
| Asian | 22/41 (54) | 16/34 (47) |
| All Other combined | 9/16 (56) | 4/6 (66) |
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved TALTZ based on the on evidence from three clinical trials of 1958 patients with moderate to severe psoriasis. The trials were conducted in the USA, Canada, Europe, Russia, Mexico, Chile, Argentina, Japan and Australia.
The figure below summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical trial data
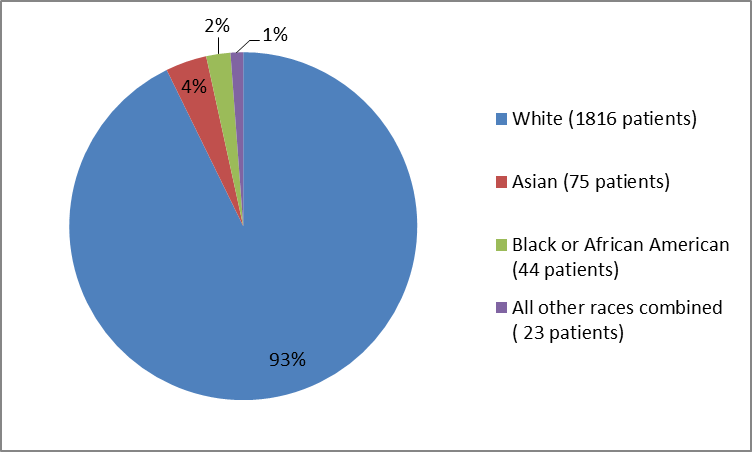
Figure 2 and Table 1 below summarize the percentage of patients by race enrolled in the clinical trials.
Figure 2. Baseline Demographics by Race
Clinical trial data
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 1816 | 93 |
| Asian | 75 | 4 |
| Black or African American | 44 | 2 |
| America Indian or Alaska Native | 9 | less than 1 |
| Native Hawaiian or Other Pacific Islander | 5 | less than 1 |
| Multiple | 8 | less than 1 |
Clinical trial data
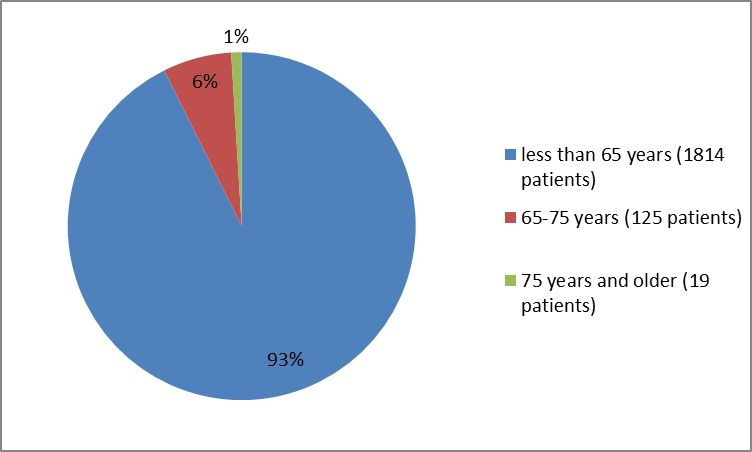
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of participants in the clinical trials.
Table 9. Baseline Demographics of Patients in the Clinical Trials (safety population)
| Demographic Parameter | Treatment Groups | Total N=1958 n (%) | |
|---|---|---|---|
| Placebo N=791 n (%) | TALTZ N=1167 n (%) | ||
| Sex | |||
| Age | |||
| Age Group | |||
| Race1 | |||
| Ethnicity | |||
| Region | |||
| Male | 559 (71) | 766 (66) | 1325 (68) |
| Female | 232 (30) | 401(34) | 633 (32) |
| Mean years (SD) | 46 (13) | 45 (13) | 45 (13) |
| Median (years) | 47 | 45 | 46 |
| Min, Max (years) | 18, 79 | 17, 84 | 17, 84 |
| <65> | 731 (92) | 1083 (93) | 1814 (93) |
| 65 years-75 years | 49 (6) | 76 (7) | 125 (6) |
| ≥75 years | 11 (1) | 8 (1) | 19 (1) |
| White | 725 (92) | 1091 (94) | 1816 (93) |
| Black or African American | 26 (3) | 18 (2) | 44 (2) |
| Asian | 34 (4) | 41 (4) | 75 (4) |
| American Indian or Alaska Native | 4 (1) | 5 (<> | 9 (<> |
| Native Hawaiian or Other Pacific Islander | 1(<> | 4 (<> | 5 (<> |
| Multiple | 1 (<> | 7 (1) | 8 (<> |
| Hispanic or Latino | 50 (6) | 93 (8) | 143 (7) |
| Not Hispanic or Latino | 613 (78) | 881 (76) | 1494 (76) |
| Unknown | 128 (16) | 193 (17) | 321 (16) |
| North America | 403 (51) | 595 (51) | 998 (51) |
| Other | 388 (49) | 572 (49) | 960 (49) |
1based on N=1166 for TALTZ
Clinical trial data
How were the trials designed?
The benefit and side effects of TALTZ were evaluated in three clinical trials of patients with moderate to severe psoriasis. In all three trials participants received treatment with either TALTZ or placebo. Some participants in Trials 2 and 3 also received drug approved for psoriasis called etanercept. Neither the participants nor the health care providers knew which treatment was being given until after the first 12 weeks of the trials were completed.
Participants were evaluated for improvement of psoriasis after 12 weeks of treatment. Some participants continued on the treatment for 12 months to determine how long the benefits will last.
How were the trials designed?
The safety and efficacy of TALTZ were established in 3 randomized, double-blind, placebo-controlled trials (Trials 1, 2 and 3) of 12 weeks duration. In the two active comparator trials (Trials 2 and 3), subjects were also randomized to receive U.S. approved etanercept for 12 weeks.
All participants had moderate to severe plaque psoriasis defined as a minimum body surface area involvement of 10%, a static Physician Global Assessment (sPGA) score of ≥3, a Psoriasis Area and Severity Index (PASI) score ≥12, and were candidates for phototherapy or systemic therapy.
The primary efficacy outcome measures for all three trials were the co-primary endpoints of PASI 75 and sPGA of “0” (clear) or “1” (minimal) with at least a 2-point improvement from the baseline.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION