Drug Trials Snapshots: STRENSIQ
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the STRENSIQ Prescribing Information for complete information.
STRENSIQ (asfotase alfa)
Stren-zeek
Alexion
Approval date: October 23, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
STRENSIQ is a drug that treats perinatal/infantile-, and juvenile-onset hypophosphatasia, referred to as HPP. HPP is a rare, genetic, progressive, metabolic disease in which patients experience devastating effects on multiple systems in the body, leading to severe disability and life-threatening complications.
STRENSIQ was studied in patients with a form of HPP that is seen in newborns (called perinatal/infantile HPP) as well as a form seen in childhood (called juvenile-onset HPP).
How is this drug used?
STRENSIQ is given as an injection under the skin (subcutaneously) three or six times per week.
What are the benefits of this drug?
Patients with perinatal/infantile-onset HPP treated with STRENSIQ had improved overall survival. These patients also had improved survival without the need for a ventilator (called ventilator-free survival). These improvements were seen when patients with HPP who were treated with STRENSIQ were compared to a group of HPP patients who did not receive treatment but were followed to study the course of their disease (called a natural history control group).
Patients with juvenile-onset HPP treated with STRENSIQ showed improvements in growth and bone health compared to a natural history control group.
What are the benefits of this drug (results of trials used to assess efficacy)?
Perinatal/Infantile Population
The table and figure below summarize efficacy results in the perinatal/infantile-onset population.
Table 3. Survival and Invasive Ventilation-Free Survival in STRENSIQ-Treated versus Historical Control Patients with Perinatal/ Infantile-Onset HPP
| STRENSIQ-Treated | Historical Control | |
|---|---|---|
| Survival | n = 68 | n = 48 |
| Alive at Point of Last Contact (%) | 91 | 27 |
| Hazard Ratio (STRENSIQ/Historical Control), 95% Confidence Interval* | 0.14 (0.05, 0.39) | |
| Kaplan-Meier Estimate and Alive at Age 1 Year (Week 48) (%) | 97 | 42 |
| Invasive Ventilation-Free Survival** | n = 54 | n = 48 |
| Alive and Not on Ventilation at Point of Last Contact (%) | 85 | 25 |
| Hazard Ratio (STRENSIQ/Historical Control), 95% Confidence Interval* | 0.21 (0.09, 0.51) | |
| Kaplan-Meier Estimate of Alive and Not on Ventilation at Age 1 Year (Week 48) (%) | 96 | 31 |
* Adjusted for year of diagnosis.
** Alive and not initiating invasive ventilation after start of STRENSIQ treatment. STRENSIQ-treated patients on invasive ventilation at baseline were excluded from this analysis.
STRENSIQ Prescribing Information, Table 7
Figure 5. Overall Survival in STRENSIQ-Treated versus Historical Control Patients with Perinatal/Infantile-Onset HPP
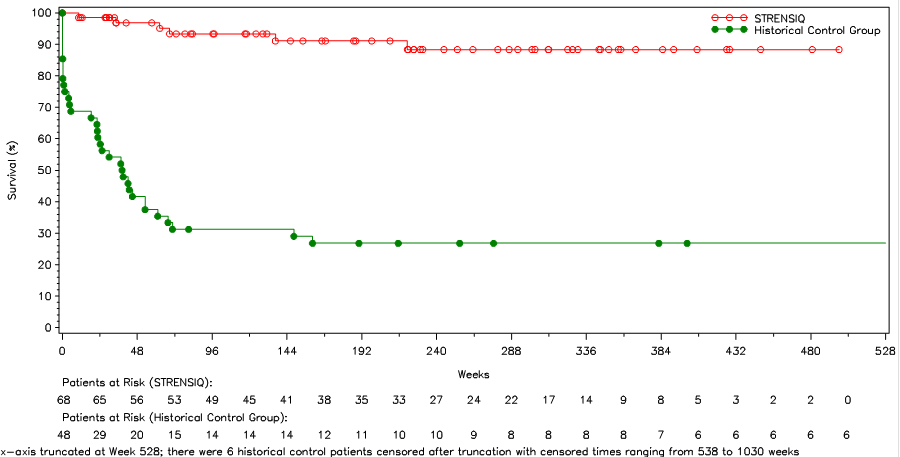
STRENSIQ Prescribing Information
Additional data regarding skeletal manifestations and growth can be found in STRENSIQ Prescribing Information.
Juvenile Population
The table below summarizes growth data from the juvenile population.
Table 4. Juvenile-Onset HPP Height and Weight Measurements as Measured by Z-Score
| Height Z-score | Weight Z-score | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Last Assessment | Baseline | Last Assessment | |||||
| Mean | Min, Max | Mean | Min, Max | Mean | Min, Max | Mean | Min, Max | |
STRENSIQ (N=8)* | -1.5 | -3.8, 0 | -0.9 | -2, 0 | -1.1 | -3.5, 2.3 | 0 | -1.3, 2.2 |
Control (N=32)** | -1.1 | -4.9, 2.6 | -1.1 | -4.9, 1.8 | -1.2 | -5, 2.1 | -1 | -5.7, 2.1 |
* The mean time interval between baseline and last assessment was 55 months (range was 53 months to 60 months).
** The mean time interval between baseline and last assessment was 61 months (range was 19 months to 109 months).
STRENSIQ Prescribing Information, Table 9
Additional efficacy data regarding skeletal manifestations and gait/mobility can be found in STRENSIQ Prescribing Information.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Perinatal/Infantile Population
- Sex: In the perinatal/infantile population, STRENSIQ worked similarly in boys and girls.
- Race: The majority of patients were white. Differences in how well STRENSIQ worked among races could not be determined.
- Age: All patients were between the ages of 1 day and 78 months. Differences in how well STRENSIQ worked among age groups could not be determined.
Juvenile Population
- Sex: There were too few patients to determine whether there were differences in how well STRENSIQ worked in boys and girls.
- Race: There were too few patients to determine whether there were differences in how well STRENSIQ worked among races.
- Age: All patients were between the ages of 6 and 12 years old. Differences in how well STRENSIQ worked among age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
In the clinical trials that included perinatal/infantile-onset HPP patients, subgroup analysis for age was not done because all patients were infants and toddlers at the time of enrollment. Subgroup analysis for race was not done because the majority of patients were white. The table below summarizes results for the clinical trials, by sex, in the perinatal/infantile population.
Table 5. Overall Survival Subgroup Analysis by Sex-- ENB-002-08/ENB-003-08 and ENB-010-10 vs. ENB-011-10 (All Qualified Enrolled/Extracted)
| ENB-002-08/ENB-003-08 and ENB-010-10 Asfotase Alfa (N = 68) | ENB-011-10 Historical Control (N = 48) | |
|---|---|---|
| Female | ||
| n | 37 | 22 |
| Alive at Point of Last Contact – n (%) | 35 (94.6%) | 7 (31.8%) |
| Corresponding 95% CI [1] | (81.8%, 99.3%) | (13.9%, 54.9%) |
| Time to Death from Birth (in Days) | ||
| n | 37 | 22 |
| Mean (SD) | 1433.5 (957.61) | 1316.0 (2141.93) |
| Median | 1181.0 | 304.5 |
| Min, Max | 81, 3367* | 1, 7211* |
| Hazard Ratio (Asfotase Alfa / Historical Control) | 0.054 | |
| Corresponding 95% CI | (0.012, 0.237) | |
| Log-Rank test p-value [2] | > | |
| Male | ||
| n | 31 | 26 |
| Alive at Point of Last Contact – n (%) | 27 (87.1%) | 6 (23.1%) |
| Corresponding 95% CI [1] | (69.5%, 97.8%) | (9.0%, 43.7%) |
| Time to Death from Birth (in Days) | ||
| n | 31 | 26 |
| Mean (SD) | 1354.0 (952.69) | 941.4 (1674.40) |
| Median | 1389.0 | 236.0 |
| Min, Max | 73, 3487* | 1, 6649* |
| Hazard Ratio (Asfotase Alfa / Historical Control) | 0.125 | |
| Corresponding 95% CI | (0.047, 0.337) | |
| Log-Rank test p-value [2] | > | |
Source: Reviewer’s Table generated from ISE ADTTE dataset.
Note: Denominators for percentages are n. * denotes censoring.
[1]: Using the Clopper-Pearson method.
[2]: Considered exploratory.
FDA Statistical Analysis
Table 6. Invasive Ventilator-Free Survival Subgroup Analysis by Sex-- ENB-002-08/ENB-003-08 and ENB-010-10 vs. ENB-011-10 (All Qualified Enrolled/Extracted)
| ENB-002-08/ENB-003-08 and ENB-010-10 Asfotase Alfa (N = 54) [3] | ENB-011-10 Historical Control (N = 48) | |
|---|---|---|
| Female | ||
| n | 31 | 22 |
| Alive and Not receiving Invasive Ventilation at Point of Last Contact – n (%) | 27 (87.1%) | 6 (27.3%) |
| Corresponding 95% CI [1] | (69.5%, 97.8%) | (10.7%, 50.2%) |
| Time to Death from Birth (in Days) | ||
| n | 31 | 22 |
| Mean (SD) | 1324.8 (980.44) | 997.2 (1809.62) |
| Median | 1078.0 | 236.0 |
| Min, Max | 81, 3366* | 1, 7211* |
| Hazard Ratio (Asfotase Alfa / Historical Control) | 0.136 | |
| Corresponding 95% CI | (0.049, 0.377) | |
| Log-Rank test p-value [2] | 0.0001 | |
| Male | ||
| n | 23 | 26 |
| Alive and Not on Ventilation at Point of Last Contact – n (%) | 19 (82.6%) | 6 (23.1%) |
| Corresponding 95% CI [1] | (61.2%, 95.1%) | (9.0%, 43.7%) |
| Time to Death from Birth (in Days) | ||
| n | 23 | 26 |
| Mean (SD) | 1513.7 (974.28) | 874.2 (1685.75) |
| Median | 1552.0 | 236.0 |
| Min, Max | 50, 3487* | 1, 6649* |
| Hazard Ratio (Asfotase Alfa / Historical Control) | 0.120 | |
| Corresponding 95% CI | (0.040, 0.359) | |
| Log-Rank test p-value [2] | 0.0001 | |
Source: Reviewer’s Table generated from ISE ADTTE dataset.
Note: Denominators for percentages are n. * denotes censoring.
[1]: Using the Clopper-Pearson method.
[2]: Considered exploratory.
[3]: Patients who were alive and not receiving invasive ventilatory support after start of STRENSIQ treatment. STRENSIQ-treated patients receiving invasive ventilation at baseline were excluded from this analysis.
FDA Statistical Analysis
What are the possible side effects?
The most common side effects in patients treated with STRENSIQ were reactions at the site of the injection (such as pain, swelling, or redness), allergic reactions, lipodystrophy (a loss of fat tissue resulting in an indentation in the skin or a thickening of fat tissue resulting in a lump under the skin) at the injection site. Calcium deposits in the eyes and kidneys were observed but it is not entirely clear if they are related to the treatment or to HPP itself.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred at a rate of at least 10% in clinical trials following subcutaneous injection of STRENSIQ, by patient population and STRENSIQ dosage regimen.
Table 7. Adverse Reactions Reported in at Least 10% of Patients with Perinatal/Infantile- or Juvenile-onset HPP Enrolled in STRENSIQ Clinical Trials
| Perinatal/ Infantile-onset HPP | Juvenile-onset HPP | |||
|---|---|---|---|---|
| Adverse Reaction Category or Term | STRENSIQ less than or equal to 6 mg/kg per week (N=66) n (%) | STRENSIQ more than 6 mg/kg/week(a) (N=13) n (%) | Total (N=79) n (%) | (N=20) n (%) |
| Injection site reactions | 38 (58) | 6 (46) | 44 (56) | 18 (90) |
| Erythema | 29 (44) | 3 (23) | 32 (41) | 15 (75) |
| Discoloration/ Hypopigmentation | 11 (17) | 1 (8) | 12 (15) | 8 (40) |
| Pain/ Tenderness | 10 (15) | 1 (8) | 11 (14) | 8 (40) |
| Pruritus/ Itching | 10 (15) | 0 (0) | 10 (13) | 7 (35) |
| Swelling | 8 (12) | 0 (0) | 8 (10) | 6 (30) |
| Induration | 9 (14) | 1 (8) | 10 (13) | 3 (15) |
| Macule | 4 (6) | 0 (0) | 4 (5) | 7 (35) |
| Reaction, not otherwise specified | 6 (9) | 1 (8) | 7 (9) | 4 (20) |
| Bruising | 6 (9) | 0 (0) | 6 (8) | 4 (20) |
| Nodule | 2 (3) | 0 (0) | 2 (3) | 2 (10) |
| Other injection site reactions(b) | 10 (15) | 3 (23) | 13 (17) | 4 (20) |
| Ectopic calcifications | 3 (5) | 0 (0) | 3 (4) | 11 (55) |
| Lipodystrophy | 12 (18) | 2 (15) | 14 (18) | 14 (70) |
| Injection site atrophy | 4 (6) | 2 (15) | 6 (8) | 8 (40) |
| Injection site hypertrophy | 5 (8) | 0 (0) | 5 (6) | 6 (30) |
| Other lipodystrophy(c) | 4 (6) | 0 (0) | 4 (5) | 1 (5) |
| Hypersensitivity reactions | 7 (11) | 3 (23) | 10 (13) | 2 (10) |
| Vomiting/Emesis | 2 (3) | 2 (15) | 4 (5) | 2 (10) |
| Other hypersensitivity reactions(d) | 6 (9) | 2 (15) | 8 (10) | 2 (10) |
a Adverse reactions are from the combined period of 6 mg/kg and above (i.e. total drug exposure regardless of starting dose and intermediary doses as long as the patient reached doses > 6 mg/kg).
b Other injection site reactions include injection site rash, inflammation, papule, hemorrhage, hematoma, urticaria, warmth, calcification, mass, scar and cellulitis.
c Other lipodystrophy includes lipohypertrophy.
d Other hypersensitivity reactions include erythema/redness, pyrexia/fever, irritability, nausea, pain, rigor/chills, hypoesthesia oral, headache, flushing, and anaphylaxis.
STRENSIQ Prescribing Information, Table 4
Were there any differences in side effects among sex, race and age?
For both perinatal/infantile and juvenile populations:
- Sex: There were too few patients to determine whether there were differences in side effects between boys and girls.
- Race: There were too few patients to determine whether there were differences in side effects among races.
- Age: All patients were between the ages of 1 day and 12 years old. Differences in side effects among age groups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The clinical trials included too few patients to assess for differences in side effects among subgroups.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The benefits and side effects of STRENSIQ were evaluated in 99 patients with perinatal/ infantile-onset HPP (disease is present at birth or soon thereafter) or juvenile-onset HPP who received treatment for up to 6.5 years during four clinical trials. In these trials, all patients received STRENSIQ and were followed during treatment.
The perinatal/infantile population included 79 patients, and the juvenile population included 20 patients. The demographics for sex and race of these 2 groups are presented separately below. Since all patients were infants and children, a summary of age groups is not presented.
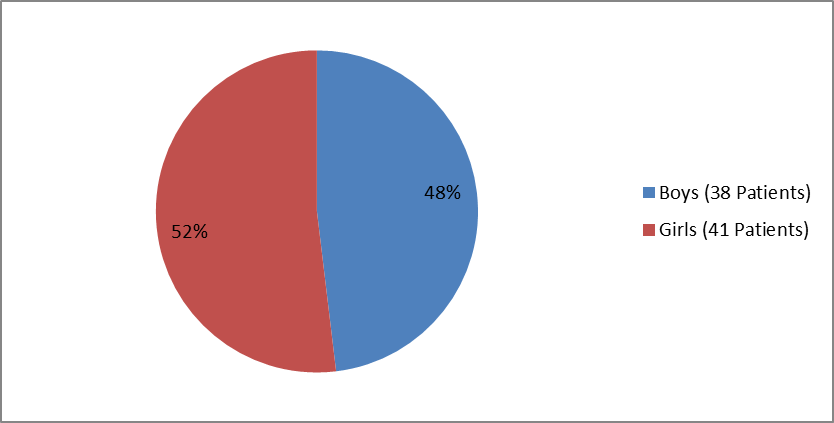
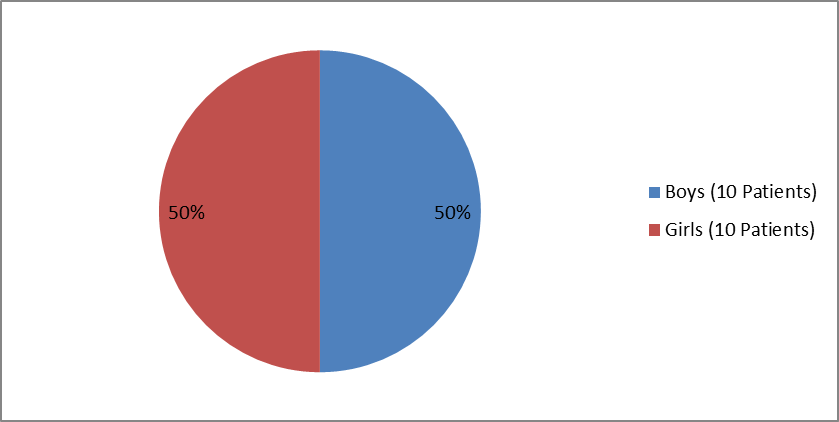
Figures 1 and 2 summarize how many boys and girls were enrolled in the clinical trials of the perinatal/infantile population and juvenile population, respectively.
Figure 1. Baseline Demographics by Sex, Perinatal/Infantile-Onset HPP
Clinical Trial Data
Figure 2. Baseline Demographics by Sex, Juvenile-Onset HPP
Clinical Trial Data
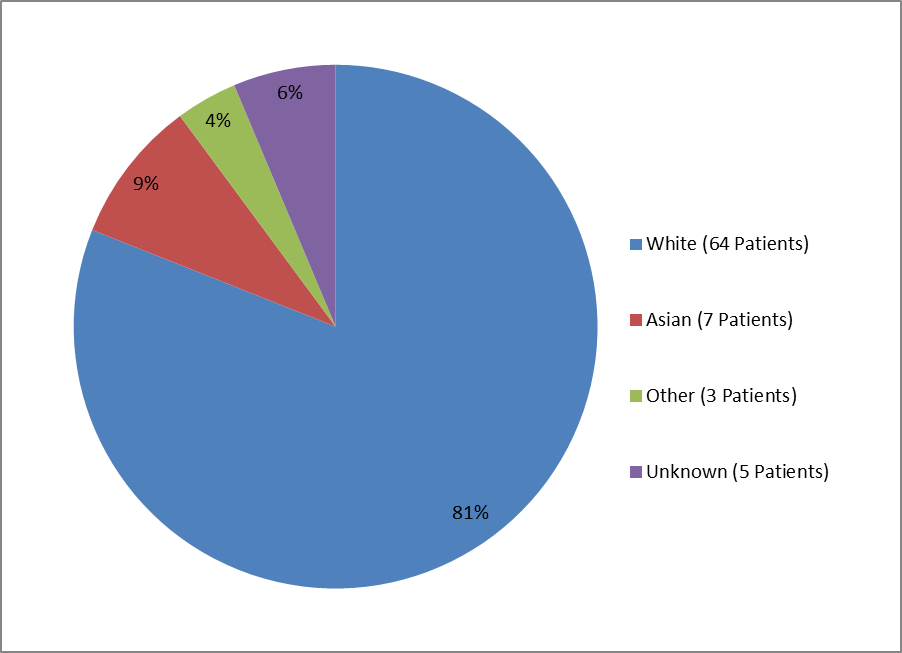
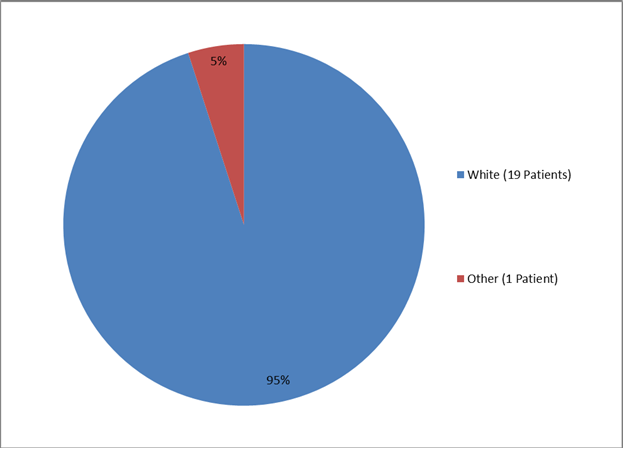
The two figures and two tables below summarize by race the percentage of patients enrolled in the clinical trials for the two populations.
Figure 3. Baseline Demographics by Race, Perinatal/Infantile-Onset HPP
Clinical Trial Data
Table 1. Baseline Demographics by Race, Perinatal/Infantile-Onset HPP
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 64 | 81% |
| Asian | 7 | 9% |
| Other | 3 | 4% |
| Unknown | 5 | 6% |
Clinical Trial Data
Figure 4. Baseline Demographics by Race, Juvenile-Onset HPP
Clinical Trial Data
Table 2. Baseline Demographics by Race, Juvenile-Onset HPP
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 19 | 95% |
| Other | 1 | 5% |
Clinical Trial Data
Who participated in the trials?
The tables below summarize demographics for the two patient populations.
Table 8. Baseline Demographics: Pooled Perinatal/Infantile Population
| Enrolled Patients (N=79) n (%) | ||
|---|---|---|
| Sex | ||
| Male | 38 (48.1) | |
| Female | 41 (51.9) | |
| Race | ||
| White | 64 (81.0) | |
| Black or African American | 0 (0.0) | |
| Asian | 7 (8.9) | |
| American Indian or Alaska Native | 0 (0.0) | |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | |
| Other | 3 (3.8) | |
| Unknown | 5 (6.3) | |
| Ethnicity | ||
| Hispanic or Latino | 2 (2.5) | |
| Not Hispanic or Latino | 72 (91.1) | |
| Unknown | 5 (6.3) | |
| Region | ||
| United States/Canada | 45 (57.0) | |
| United States | 30 (38.0) | |
| Canada | 15 (19.0) | |
| Europe | 26 (32.9) | |
| Asia | 5 (6.3) | |
| Rest of World | 3 (3.8) | |
Clinical Trial Data
Table 9. Baseline Demographics: Juvenile Population
| Enrolled Population (N=20) n (%) | ||
|---|---|---|
| Sex | ||
| Male | 10 (50.0) | |
| Female | 10 (50.0) | |
| Race | ||
| White | 19 (95.0) | |
| Black or African American | 0 (0.0) | |
| Asian | 0 (0.0) | |
| American Indian or Alaska Native | 0 (0.0) | |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | |
| Other | 1 (5.0) | |
| Ethnicity | ||
| Hispanic or Latino | 1 (5.0) | |
| Not Hispanic or Latino | 19 (95.0) | |
| Region | ||
| United States/Canada | 20 (100.0) | |
| United States | 14 (70.0) | |
| Canada | 6 (30.0) | |
| Europe | 0 (0.0) | |
| Asia | 0 (0.0) | |
| Rest of World | 0 (0.0) | |
Clinical Trial Data
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.