Drug Trials Snapshots: STIOLTO RESPIMAT
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the STIOLTO RESPIMAT Prescribing Information for complete information.
STIOLTO RESPIMAT (tiotropium bromide and olodaterol)
(sti-OL-to– RES peh mat)
Boehringer Ingelheim
Approval date: May 21, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
STIOLTO RESPIMAT is a long-term treatment for adults with a lung disease called COPD (chronic obstructive pulmonary disease). It is a combination of two drugs already used for COPD.
STIOLTO RESPIMAT should only be used to treat COPD and not for other breathing problems.
How is this drug used?
STIOLTO RESPIMAT is a spray inhaled through the mouth. Patients should use 2 puffs once a day at the same time each day.
What are the benefits of this drug?
STIOLTO RESPIMAT relaxes the muscles in the airways. This allows air to move easier in the lungs which helps prevent symptoms such as wheezing, cough, chest tightness, and shortness of breath.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 below summarizes the co-primary efficacy endpoints for each trial separately. These were based on the FEV1 response for STIOLTO RESPIMAT compared to tiotropium 5 mcg and olodaterol 5 mcg after 24 weeks.
Table 2. FEV1 AUC0-3hr and Trough FEV1 response for STIOLTO RESPIMAT compared to tiotropium 5 mcg and olodaterol 5 mcg
| Trial 1 | Trial 2 | |||||
|---|---|---|---|---|---|---|
| n | Mean (L) | Difference (L) (95%CI) | n | Mean (L) | Difference (L) (95%CI) | |
| FEV1 AUC0-3h response | ||||||
| STIOLTO RESPIMAT | 522 | 0.256 | _ | 502 | 0.268 | _ |
| Tiotropium 5 mcg | 526 | 0.139 | 0.117 (0.094,0.140 | 500 | 0.165 | 0.103 (0.078,0.127) |
| Olodaterol 5 mcg | 525 | 0.133 | 0.123 (0.100,0.146) | 507 | 0.136 | 0.132 (0.108, 0.157) |
| Trough FEV1 response | ||||||
| STIOLTO RESPIMAT | 521 | 0.136 | _ | 497 | 0.145 | _ |
| Tiotropium 5 mcg | 520 | 0.065 | 0.071 (0.047, 0.094) | 498 | 0.096 | 0.050 (0.024, 0.075) |
| Olodaterol 5 mcg | 519 | 0.054 | 0.082 (0.059, 0.106) | 503 | 0.057 | 0.088 (0.063, 0.113) |
L=liters; Pre-treatment baseline FEV1: Trial 1=1.16 L; Trial 2=1.15 L
p≤0.0001 for all comparisons between STIOLTO RESPIMAT and the monotherapies
Source: STIOLTO RESPIMAT Prescribing Information, Section 14, Table 2
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The majority of patients in the trials were men. STIOLTO RESPIMAT appeared to be similarly effective in men and women.
- Race: The majority of patients in the trials were white or Asian. STIOLTO RESPIMAT appears to be similarly effective in these races. Differences in response to STIOLTO RESPIMAT in other races could not be determined.
- Age: All patients in the trial were 40 years of age and above. STIOLTO RESPIMAT was similarly effective in all age groups studied.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize the responses to STIOLTO RESPIMAT compared to tiotropium 5 mcg and olodaterol for the populations in each trial.
Table 3. Subgroup Analysis of Primary Endpoint (FEV1 AUC0-3h response) STIOLTO RESPIMAT v. Tiotropium
| Subgroup | Trial 1 | Trial 2 | ||||
|---|---|---|---|---|---|---|
| SR v. Tio (L) | 95% CI | SR v. Tio (L) | 95% CI | |||
| LL | UL | LL | UL | |||
| Sex | ||||||
| Male | 0.118 | 0.09 | 0.145 | 0.097 | 0.066 | 0.127 |
| Female | 0.114 | 0.076 | 0.152 | 0.118 | 0.078 | 0.158 |
| Age Group | ||||||
| >=17 - 65> | 0.142 | 0.106 | 0.177 | 0.099 | 0.062 | 0.135 |
| >=65 years | 0.091 | 0.057 | 0.125 | 0.123 | 0.087 | 0.159 |
| >=75 years | 0.12 | 0.066 | 0.174 | 0.024 | -0.036 | 0.084 |
| Race | ||||||
| White | 0.115 | 0.087 | 0.143 | 0.112 | 0.082 | 0.142 |
| Black or African American | NE | NE | NE | NE | NE | NE |
| Asian | 0.123 | 0.079 | 0.166 | 0.089 | 0.046 | 0.132 |
| American Indian or Alaska Native | NE | NE | NE | NE | NE | NE |
| Unknown | NE | NE | NE | NE | NE | NE |
| Region | ||||||
| United States | 0.086 | 0.03 | 0.141 | 0.126 | 0.068 | 0.183 |
| Rest of the World | 0.123 | 0.098 | 0.147 | 0.097 | 0.07 | 0.125 |
SR= STIOLTO RESPIMAT
Tio= Tiotropium 5mcg
NE=Not evaluable (too few patients to perform subgroup analysis)
L= liters
Source: Company Clinical Trial Data
Table 4. Subgroup Analysis of Primary Endpoint (trough FEV1 response) STIOLTO RESPIMAT v. Tiotropium
| Subgroup | Trial 1 | Trial 2 | ||||
|---|---|---|---|---|---|---|
| SR v. Tio (L) | 95% CI | SR v. Tio (L) | 95% CI | |||
| LL | UL | LL | UL | |||
| Sex | ||||||
| Male | 0.071 | 0.043 | 0.1 | 0.05 | 0.019 | 0.081 |
| Female | 0.066 | 0.028 | 0.105 | 0.05 | 0.009 | 0.091 |
| Age Group | ||||||
| >=17 - 65> | 0.088 | 0.051 | 0.124 | 0.053 | 0.016 | 0.091 |
| >=65 years | 0.059 | 0.026 | 0.093 | 0.06 | 0.023 | 0.097 |
| >=75 years | 0.04 | -0.017 | 0.098 | -0.019 | -0.082 | 0.044 |
| Race | ||||||
| White | 0.064 | 0.036 | 0.093 | 0.057 | 0.026 | 0.087 |
| Black or African American | NE | NE | NE | NE | NE | NE |
| Asian | 0.088 | 0.044 | 0.131 | 0.036 | -0.01 | 0.082 |
| American Indian or Alaska Native | NE | NE | NE | NE | NE | NE |
| Unknown | NE | NE | NE | NE | NE | NE |
| Region | ||||||
| United States | 0.021 | -0.035 | 0.076 | 0.083 | 0.024 | 0.143 |
| Rest of the World | 0.08 | 0.054 | 0.106 | 0.042 | 0.014 | 0.07 |
SR= STIOLTO RESPIMAT
Tio= Tiotropium 5mcg
NE=Not evaluable (too few patients to perform subgroup analysis)
L=liters
Source: Company Clinical Trial Data
Table 5. Subgroup Analysis of Primary Endpoint (FEV1 AUC0-3h response) STIOLTO RESPIMAT v. Olodaterol
| Subgroup | Trial 1 | Trial 2 | ||||
|---|---|---|---|---|---|---|
| SR v. Olo (L) | 95% CI | SR v. Olo (L) | 95% CI | |||
| LL | UL | LL | UL | |||
| Sex | ||||||
| Male | 0.122 | 0.093 | 0.15 | 0.127 | 0.097 | 0.157 |
| Female | 0.125 | 0.087 | 0.163 | 0.147 | 0.106 | 0.187 |
| Age Group | ||||||
| >=17 - 65> | 0.154 | 0.119 | 0.189 | 0.148 | 0.111 | 0.186 |
| >=65 years | 0.085 | 0.051 | 0.119 | 0.111 | 0.076 | 0.147 |
| >=75 years | 0.147 | 0.089 | 0.206 | 0.088 | 0.03 | 0.146 |
| Race | ||||||
| White | 0.111 | 0.082 | 0.139 | 0.13 | 0.1 | 0.159 |
| Black or African American | NE | NE | NE | NE | NE | NE |
| Asian | 0.155 | 0.112 | 0.198 | 0.144 | 0.1 | 0.188 |
| American Indian or Alaska Native | NE | NE | NE | NE | NE | NE |
| Unknown | NE | NE | NE | NE | NE | NE |
| Region | ||||||
| United States | 0.108 | 0.051 | 0.165 | 0.151 | 0.094 | 0.208 |
| Rest of the World | 0.125 | 0.1 | 0.15 | 0.128 | 0.101 | 0.155 |
SR= STIOLTO RESPIMAT
Olo=Olodaterol 5 mcg
NE=Not evaluable (too few patients to perform subgroup analysis)
L=liters
Source: Company Clinical Trial Data
Table 6. Subgroup Analysis of Primary Endpoint (trough FEV1 response) STIOLTO RESPIMAT v. Olodaterol
| Subgroup | Trial 1 | Trial 2 | ||||
|---|---|---|---|---|---|---|
| SR v. Olo (L) | 95% CI | SR v. Olo (L) | 95% CI | |||
| LL | UL | LL | UL | |||
| Sex | ||||||
| Male | 0.088 | 0.059 | 0.117 | 0.09 | 0.059 | 0.121 |
| Female | 0.067 | 0.029 | 0.106 | 0.083 | 0.041 | 0.125 |
| Age Group | ||||||
| >=17 - 65> | 0.096 | 0.06 | 0.133 | 0.105 | 0.066 | 0.144 |
| >=65 years | 0.062 | 0.028 | 0.096 | 0.065 | 0.028 | 0.102 |
| >=75 years | 0.106 | 0.043 | 0.167 | 0.073 | 0.012 | 0.134 |
| Race | ||||||
| White | 0.062 | 0.033 | 0.091 | 0.087 | 0.057 | 0.118 |
| Black or African American | NE | NE | NE | NE | NE | NE |
| Asian | 0.127 | 0.084 | 0.17 | 0.098 | 0.051 | 0.144 |
| American Indian or Alaska Native | NE | NE | NE | NE | NE | NE |
| Unknown | NE | NE | NE | NE | NE | NE |
| Region | ||||||
| United States | 0.064 | 0.007 | 0.12 | 0.107 | 0.048 | 0.166 |
| Rest of the World | 0.085 | 0.059 | 0.111 | 0.083 | 0.055 | 0.111 |
SR= STIOLTO RESPIMAT; Olo=Olodaterol 5 mcg
NE=Not evaluable (too few patients to perform subgroup analysis)
L=liters
Source: Company Clinical Trial Data
What are the possible side effects?
The most common side effects of STIOLTO RESPIMAT include common cold, cough, and back pain.
STIOLTO RESPIMAT should never be used to treat asthma. Using STIOLTO RESPIMAT in someone with asthma can worsen the asthma and lead to death.
STIOLTO RESPIMAT can cause serious side effects, including:
- Severe allergic reactions, including rash, itchy and raised patches of skin, breathing problems, and swelling of the mouth, face, and tongue
- Sudden shortness of breath that may be life-threatening
- Heart problems including fast or irregular heartbeat, chest pain, and an increase in blood pressure
- New or worsening eye problem called narrow-angle glaucoma: symptoms can include eye pain, blurry vision, red eyes and seeing halos or colored images around lights
- New or worsening problems with urination: symptoms can include difficulty passing urine or painful urination
- Low blood potassium
- High blood sugar
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions for the two pooled trials. The population represented is the Safety population, which includes any patient who received at least one dose of trial drug.
Table 7. Number and Frequency of Adverse Drug Reactions in STIOLTO RESPIMAT arm Greater than 3% and Higher than Tiotropium and/or Olodaterol (Pooled data from the trials)
| Adverse Drug Reaction | STIOLTO RESPIMAT (once daily) n=1029 n (%) | Tiotropium (5 mcg once daily) n=1033 n (%) | Olodaterol (5 mcg once daily) n=1038 n (%) |
|---|---|---|---|
| Nasopharyngitis | 128 (12.4) | 121 (11.7) | 131 (12.6) |
| Cough | 40 (3.9) | 45 (4.4) | 31 (3.0) |
| Back Pain | 37 (3.6) | 19 (1.8) | 35 (3.4) |
Source: STIOLTO RESPIMAT Prescribing Information, Section 6, Table 1
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race, and age.
- Sex: The majority of patients in the trials were men. The risk of overall side effects appeared to be similar in men and women.
- Race: The majority of patients in the trials were white or Asian. The risk of overall side effects appeared to be similar in these races. Differences in side effects in other races could not be determined.
- Age: All patients in the trial were 40 years of age and above. The risk of overall side effects was similar in all age groups studied.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events in the two trials by subgroups.
Table 8. Adverse Events During Treatment in the Pooled Trials
| Subgroup | STIOLTO RESPIMAT | Tiotropium 5 mcg | Olodaterol 5 mcg | |||
|---|---|---|---|---|---|---|
| x (%)* | Total, n | x (%)* | Total, n | x (%)* | Total, n | |
| Any TEAEs** | 761 (74.0) | 1029 | 757 (73.3) | 1033 | 795 (76.6) | 1038 |
| Sex | ||||||
| Male | 524 (71.5) | 733 | 534 (70.7) | 755 | 573 (75.0) | 764 |
| Female | 237 (80.1) | 296 | 223 (80.2) | 278 | 222 (81.0) | 274 |
| Age Group | ||||||
| >=17 - 65> | 379 (72.2) | 525 | 376 (69.6) | 540 | 389 (74.8) | 520 |
| >=65 years | 305 (74.9) | 407 | 290 (75.7) | 383 | 319 (78.2) | 408 |
| >=75 years | 77 (79.4) | 97 | 91 (82.7) | 110 | 87 (79.1) | 110 |
| Race | ||||||
| White | 555 (75.8) | 732 | 537 (75.2) | 714 | 564 (77.4) | 729 |
| Black or African American | 14 (87.5) | 16 | 7 (58.3) | 12 | 10 (83.3) | 12 |
| Asian | 169 (67.6) | 250 | 190 (68.3) | 278 | 210 (75.3) | 279 |
| American Indian or Alaska Native | 6 (85.7) | 7 | 11 (91.7) | 12 | 1 (100.0) | 1 |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 17 (70.8) | 24 | 12 (70.6) | 17 | 10 (58.8) | 17 |
| Region | ||||||
| United States | 132 (80.0) | 165 | 130 (78.3) | 166 | 130 (79.3) | 164 |
| Rest of the World | 629 (72.8) | 864 | 627 (72.3) | 867 | 665 (76.1) | 874 |
* Percentages are calculated based on the number of subjects in the subgroup per arm
**Treatment Emergent Adverse Events
Source: Company Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved STIOLTO RESPIMAT based on evidence from two clinical trials which included 3100 adult patients with COPD who received STIOLTO RESPIMAT or one of its individual components (tiotropium or olodaterol). The trials were conducted in North America, Western Europe, East Asia, Eastern Europe, Latin America, India, Australia, New Zealand, and South Africa.
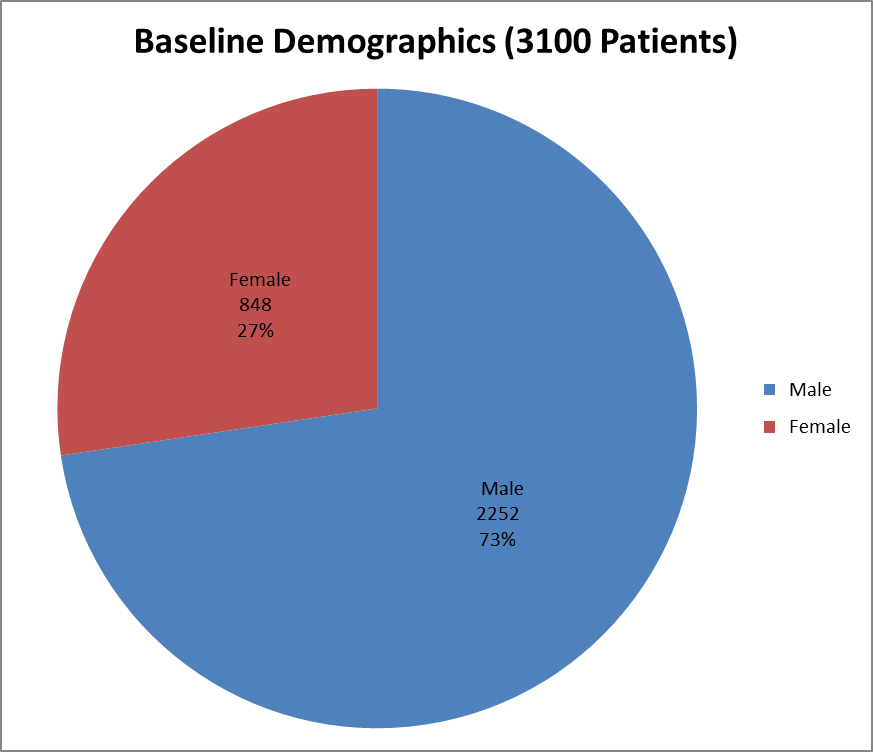
Figure 1 summarizes how many men and women were enrolled in the clinical trials.
Figure 1. Baseline Demographics by Sex
Source: Company Clinical Trial Data
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trials.
Figure 2. Baseline Demographics by Race
Source: Company Clinical Trial Data
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 2175 | 70.2% |
| Black or African American | 40 | 1.3% |
| Asian | 807 | 26.0% |
| American Indian or Alaska Native | 20 | 0.6% |
| Unknown | 58 | 1.9% |
Source: Company Clinical Trial Data
The figure below summarizes the age of patients in the clinical trial. Because COPD is rarely seen in younger adults, the trials only included patients 40 years of age and above.
Figure 3. Baseline Demographics by Age
Source: Company Clinical Trial Data
Who participated in trials?
Table 9. Baseline Demographics for the Trials
| Demographic Parameters | Trial #1 | Trial #2 | Total (N=3100) n(%) | |||||
|---|---|---|---|---|---|---|---|---|
| STIOLTO RESPIMAT (N=522) n(%)* | Tiotropium (N=527) n(%) | Olodaterol (N=528) n(%) | STIOLTO RESPIMAT (N=507) n(%) | Tiotropium (N=506) n(%) | Olodaterol (N=510) n(%) | |||
| Sex | ||||||||
| Male | 384 (73.6) | 383 (72.7) | 386(73.1) | 349 (68.8) | 372 (73.5) | 378 (74.1) | 2252 (72.6) | |
| Female | 138 (26.4) | 144 (27.3) | 142(26.9) | 158 (31.2) | 134 (26.5) | 132 (25.9) | 848 (27.4) | |
| Age | ||||||||
| Mean years (SD) | 64.8 (8.2) | 64.2 (8.5) | 63.7 (8.0) | 62.7 (8.4) | 63.5 (8.7) | 64.7 (8.3) | 64.0 (8.4) | |
| Median (years) | 65 | 64 | 64 | 64 | 64 | 65 | 64 | |
| Min, Max (years) | 42, 85 | 40, 89 | 40, 86 | 40, 82 | 40, 97 | 43, 87 | 40, 97 | |
| Age Group | ||||||||
| >=17 - 65> | 240 (46.0) | 268 (50.9) | 278(52.7) | 285 (56.2) | 272 (53.8) | 242 (47.5) | 1585 (51.1) | |
| >=65 - 75> | 223 (42.7) | 199 (37.8) | 205(38.8) | 184 (36.3) | 184 (36.4) | 203 (39.8) | 1198 (38.6) | |
| >=75 years | 59 (11.3) | 60 (11.4) | 45 (8.5) | 38 (7.5) | 50 (9.9) | 65 (12.7) | 317 (10.2) | |
| Race | ||||||||
| White | 357 (68.4) | 356 (67.6) | 358(67.8) | 375 (74.0) | 358 (70.8) | 371 (72.7) | 2175 (70.2) | |
| Black or African American | 5 (1.0) | 5 (0.9) | 4 (0.8) | 11 (2.2) | 7 (1.4) | 8 (1.6) | 40 (1.3) | |
| Asian | 132 (25.3) | 141 (26.8) | 150(28.4) | 118 (23.3) | 137 (27.1) | 129 (25.3) | 807 (26) | |
| American Indian or Alaska Native | 6 (1.1) | 9 (1.7) | 0 (0.0) | 1 (0.2) | 3 (0.6) | 1 (0.2) | 20 (0.6) | |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | |
| Unknown | 22 (4.2) | 16 (3.0) | 16 (3.0) | 2 (0.4) | 1 (0.2) | 1 (0.2) | 58 (1.9) | |
| Region | ||||||||
| United States | 73 (14.0) | 86 (16.3) | 78 (14.8) | 92 (18.1) | 80 (15.8) | 86 (16.9) | 495 (16) | |
| Rest of the World | 449 (86.0) | 441 (83.7) | 450 (85.2) | 415 (81.9) | 426 (84.2) | 424 (83.1) | 2605 (84) | |
* Percentages are calculated based on the number of subjects in the subgroup per arm
Source: Company Clinical Trial Data
How were the trials designed?
There were two trials that evaluated the benefit and side effects of STIOLTO RESPIMAT. In each trial, patients were randomly assigned to receive either STIOLTO RESPIMAT or one of its individual components (tiotropium or olodaterol) once daily for 52 weeks. The benefit of STIOLTO RESPIMAT was measured at 24 weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
The trials measured how well air flows through the lungs and compared STIOLTO RESPIMAT to each of the other two drugs.
How were the trials designed?
Two randomized, multicenter, double-blind, active controlled, trials were conducted to assess the efficacy and safety of 24 weeks of once daily treatment of orally inhaled tiotropium and olodaterol fixed dose combination (5mcg/5mcg) (delivered by the Respimat® Inhaler) in comparison to the individual components (5 mcg tiotropium, 5 mcg olodaterol delivered by the Respimat® Inhaler) in patients with chronic obstructive pulmonary disease. The trials enrolled patients with a diagnosis of moderate to severe COPD, with the following pertinent entry criteria: a) 40 years of age and older; b) FEV1 80%="" predicted;="" and="" c)="" current="" or="" ex-smokers="" with="" a="" 10="" pack-year="" smoking="">
The co-primary endpoints for the trials were FEV1 AUC0-3h and trough FEV1response at week 24. Safety assessments continued until Week 52.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION