Drug Trials Snapshots: STEGLATRO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the STEGLATRO Package Insert for complete information.
STEGLATRO (ertugliflozin)
Steh-GLA-troh
Merck Sharpe & Dohme Corp.
Approval date: December 19, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
STEGLATRO is a drug that improves blood sugar control in adults with type 2 diabetes when used in addition to diet and exercise.
How is this drug used?
STEGLATRO is a tablet that is taken by mouth once daily in the morning.
What are the benefits of this drug?
In patients with type 2 diabetes treatment with STEGLATRO can lower hemoglobin A1c (HbA1c). HbA1c is a measure of blood sugar control over the last 8-12 weeks.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of STEGLATRO as monotherapy, in addition to diet and exercise is presented in the table below. The primary efficacy endpoint was the change in HbA1c from the start of each trial.
Table 2: Results at Week 26 from a Placebo-Controlled Monotherapy Trial (NCT01958671) of STEGLATRO in Patients with Type 2 Diabetes Mellitus*
| Placebo | STEGLATRO 5 mg |
STEGLATRO 15 mg |
|
|---|---|---|---|
| HbA1c (%) | N = 153 | N = 155 | N = 151 |
| Baseline (mean) | 8.1 | 8.2 | 8.4 |
| Change from baseline (LS mean†) | -0.2 | -0.7 | -0.8 |
| Difference from placebo (LS mean†, 95% CI) | -0.6‡ (-0.8, -0.4) | -0.7‡ (-0.9, -0.4) | |
| Patients [N (%)] with HbA1c <> | 26 (16.9) | 47 (30.1) | 59 (38.8) |
| FPG (mg/dL) | N = 150 | N = 151 | N = 149 |
| Baseline (mean) | 180.2 | 180.9 | 179.1 |
| Change from baseline (LS mean†) | -11.6 | -30.1 | -36.4 |
| Difference from placebo (LS mean†, 95% CI) | -19.4‡ (-27.6, -11.2) | -24.8‡ (-33.2, -16.4) |
* N includes all randomized and treated patients with a baseline measurement of the outcome variable. At Week 26, the primary HbA1c endpoint was missing for 23%, 11%, and 16% of patients, and during the trial, rescue medication was initiated by 25%, 2%, and 3% of patients randomized to placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Missing Week 26 measurements were imputed using multiple imputation with a mean equal to the baseline value of the patient. Results include measurements collected after initiation of rescue medication. For those patients who did not receive rescue medication and had values measured at 26 weeks, the mean changes from baseline for HbA1c were -0.1%, -0.8%, and -1.0% for placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively.
† Intent-to-treat analysis using ANCOVA adjusted for baseline value, prior antihyperglycemic medication, and baseline eGFR.
‡ p<0.001 compared="" to="">
STEGLATRO Prescribing Information
The efficacy of STEGLATRO, in addition to diet and exercise, and on background metformin therapy is presented in the table below. The primary efficacy endpoint was the change in HbA1c from the start of each trial.
Table 3: Results at Week 26 from a Placebo Controlled Trial (NCT02033889) for STEGLATRO Used in Combination with Metformin in Patients with Type 2 Diabetes Mellitus*
| Placebo | STEGLATRO 5 mg | STEGLATRO 15 mg | |
|---|---|---|---|
| HbA1c (%) | N = 207 | N = 205 | N = 201 |
| Baseline (mean) | 8.2 | 8.1 | 8.1 |
| Change from baseline (LS mean†) | -0.2 | -0.7 | -0.9 |
| Difference from placebo (LS mean†, 95% CI) | -0.5‡ (-0.7, -0.4) | -0.7‡ (-0.9, -0.5) | |
| Patients [N (%)] with HbA1c <> | 38 (18.4) | 74 (36.3) | 87 (43.3) |
| FPG (mg/dL) | N = 202 | N = 199 | N = 201 |
| Baseline (mean) | 169.1 | 168.1 | 167.9 |
| Change from baseline (LS mean†) | -8.7 | -30.3 | -40.9 |
| Difference from placebo (LS mean†, 95% CI) | -21.6‡ (-27.8, -15.5) | -32.3‡ (-38.5, -26.0) |
* N includes all randomized and treated patients with a baseline measurement of the outcome variable. At Week 26, the primary HbA1c endpoint was missing for 12%, 6%, and 9% of patients, and during the trial, rescue medication was initiated by 18%, 3%, and 1% of patients randomized to placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Missing Week 26 measurements were imputed using multiple imputation with a mean equal to the baseline value of the patient. Results include measurements collected after initiation of rescue medication. For those patients who did not receive rescue medication and had values measured at 26 weeks, the mean changes from baseline for HbA1c were -0.2%, -0.7%, and -1.0% for placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively.
† Intent-to-treat analysis using ANCOVA adjusted for baseline value, prior antihyperglycemic medication, menopausal status and baseline eGFR.
‡ p<0.001 compared="" to="">
STEGLATRO Prescribing Information
The efficacy of STEGLATRO, in addition to diet and exercise, and on background metformin and sitagliptin therapy is presented in the table below. The primary efficacy endpoint was the change in HbA1c from the start of each trial.
Table 4: Results at Week 26 from an Add-on Trial (NCT02036515) of STEGLATRO in Combination with Metformin and Sitagliptin in Patients with Type 2 Diabetes Mellitus*
| Placebo | STEGLATRO 5 mg | STEGLATRO 15 mg | |
|---|---|---|---|
| HbA1c (%) | N = 152 | N = 155 | N = 152 |
| Baseline (mean) | 8.0 | 8.1 | 8.0 |
| Change from baseline (LS mean†) | -0.2 | -0.7 | -0.8 |
| Difference from placebo (LS mean†, 95% CI) | -0.5‡ (-0.7, -0.3) | -0.6‡ (-0.8, -0.4) | |
| Patients [N (%)] with HbA1c <> | 31 (20.2) | 54 (34.6) | 64 (42.3) |
| FPG (mg/dL) | N = 152 | N = 156 | N = 152 |
| Baseline (mean) | 169.6 | 167.7 | 171.7 |
| Change from baseline (LS mean†) | -6.5 | -25.7 | -32.1 |
| Difference from placebo (LS mean†, 95% CI) | -19.2‡ (-26.8, -11.6) | -25.6‡ (-33.2, -18.0) |
* N includes all randomized and treated patients with a baseline measurement of the outcome variable. At Week 26, the primary HbA1c endpoint was missing for 10%, 11%, and 7% of patients and during the trial, rescue medication was initiated by 16%, 1%, and 2% of patients randomized to placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Missing Week 26 measurements were imputed using multiple imputation with a mean equal to the baseline value of the patient. Results include measurements collected after initiation of rescue medication. For those patients who did not receive rescue medication and had values measured at 26 weeks, the mean changes from baseline for HbA1c were -0.2%, -0.8%, and -0.9% for placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively.
† Intent-to-treat analysis using ANCOVA adjusted for baseline value, prior antihyperglycemic medication and baseline eGFR.
‡ p<0.001 compared="" to="">
STEGLATRO Prescribing Information
The efficacy of STEGLATRO compared to glimepiride, in addition to diet and exercise, and on background metformin therapy is presented in the table below. The primary efficacy endpoint was the change in HbA1c from the start of each trial.
Table 5: Results at Week 52 from an Active-Controlled Trial (NCT01999218) Comparing STEGLATRO to Glimepiride as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on Metformin*
| Glimepiride | STEGLATRO 5 mg | STEGLATRO 15 mg | |
|---|---|---|---|
| HbA1c (%) | N = 437 | N = 447 | N = 440 |
| Baseline (mean) | 7.8 | 7.8 | 7.8 |
| Change from baseline (LS mean†) | -0.6 | -0.5 | -0.5 |
| Difference from glimepiride (LS mean†, 95% CI) | 0.2‡ (0.0, 0.3) | 0.1‡ (-0.0, 0.2) | |
| Patients [N (%)] with HbA1c <> | 208 (47.7) | 177 (39.5) | 186 (42.2) |
* N includes all randomized and treated patients with a baseline measurement of the outcome variable. At Week 52, the primary HbA1c endpoint was missing for 15%, 20%, and 16% of patients and during the trial, rescue medication was initiated by 3%, 6%, and 4% of patients randomized to glimepiride, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Missing Week 52 measurements were imputed using multiple imputation with a mean equal to the baseline value of the patient. Results include measurements collected after initiation of rescue medication. For those patients who did not receive rescue medication and had values measured at 52 weeks, the mean changes from baseline for HbA1c were -0.8%, -0.6%, and -0.7% for glimepiride, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively.
† Intent-to-treat analysis using ANCOVA adjusted for baseline value, prior antihyperglycemic medication and baseline eGFR.
‡ Non-inferiority is declared when the upper bound of the two-sided 95% confidence interval (CI) for the mean difference is less than 0.3%.
STEGLATRO Prescribing Information
The following trials presented below were also used to support approval of STEGLATRO:
- Active-Controlled Trial (NCT020991110) of STEGLATRO in Combination with Sitagliptin versus STEGLATRO Alone and Sitagliptin Alone, as Add-on to Metformin
A total of 1,233 patients received treatment for 26 weeks. After 26 weeks of treatment, the STEGLATRO 5 mg or 15 mg + sitagliptin 100 mg demonstrated statistically significant greater reductions in HbA1c compared to STEGLATRO (5 mg or 15 mg) alone or sitagliptin 100 mg alone. - Placebo-Controlled Trial (NCT02226003) of STEGLATRO Combination Therapy with Sitagliptin
A total of 291 patients received treatment for 26 weeks. After 26 weeks of treatment, STEGLATRO 5 mg and 15 mg, in combination with sitagliptin at 100 mg, demonstrated statistically significant reductions in HbA1c compared to placebo. - Placebo-Controlled Trial (NCT01986855) of STEGLATRO in Patients with Moderate Renal Impairment, as Add-on to Antidiabetic Therapy
A total of 468 patients with type 2 2DM, who had moderate renal impairment, received treatment for 26 weeks. Treatment with STEGLATRO did not result in statistically significant reductions in HbA1c compared to placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: STEGLATRO worked similarly in men and women.
- Race: The majority of patients were White. The number of patients of other races was limited; therefore, differences in response among races could not be determined.
- Age: STEGLATRO worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 6 below summarizes the results for the primary efficacy endpoint, the change in HbA1c by sex, age, and race for a pool of 4 of the 7 controlled trials (NCT01958671, NCT02099110, NCT02036515, and NCT02033889).
Table 6. Difference (95% Confidence Interval) in Average Change in HbA1c (STEGLATRO Minus Comparator)
| Subgroup Overall 15 mg vs. Pl. 5 mg vs. Pl. |
Estimate -0.56 -0.48 |
Lower 95% -0.65 -0.57 |
Upper 95% -0.46 -0.38 |
| Women 15 mg vs. Pl. 5 mg vs. Pl. |
-0.56 -0.50 |
-0.69 -0.63 |
-0.42 -0.37 |
| Men 15 mg vs. Pl. 5 mg vs. Pl. |
-0.57 -0.48 |
-0.70 -0.61 |
-0.43 -0.34 |
| White 15 mg vs. Pl. 5 mg vs. Pl. |
-0.53 -0.48 |
-0.65 -0.59 |
-0.43 -0.37 |
| Black 15 mg vs Pl. 5 mg vs. Pl. |
-0.40 -0.27 |
-0.81 -0.68 |
0.02 0.15 |
| Asian 15 mg vs Pl. 5 mg vs. Pl. |
-0.65 -0.54 |
-0.91 -0.80 |
-0.40 -0.28 |
| Age>65 years 15 mg vs. Pl. 5 mg vs. Pl. |
-0.51 -0.39 |
-0.70 -0.58 |
-0.32 -0.21 |
| Age < 65="" years=""> 15 mg vs. Pl. 5 mg vs. Pl. |
-0.57 -0.50 |
-0.68 -0.61 |
-0.46 -0.39 |
FDA Statistical Review
What are the possible side effects?
STEGLATRO may cause serious side effects such as decrease in blood pressure, kidney injury, surgery to remove lower limbs, increased cholesterol levels, and very low blood sugar levels when used in combination with insulin or medications that increase insulin, and serious urinary tract infection. Serious urinary tract infections can spread to the kidneys (pyeleonephritis) or cause a serious, potentially life-threatening blood infection (urosepsis).
STEGLATRO may also cause ketoacidosis. Diabetic ketoacidosis is a serious, potentially life-threatening complication that occurs when the body produces high levels of acids in the blood. The acids are called ketones.
The most common side effects with STEGLATRO are genital yeast infections.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes common adverse reactions for the safety population from the 3 placebo-controlled trials. Demographics of this population are presented in Table 10 under MORE INFO section.
Table 7. Adverse Reactions Reported in ≥2% of Patients with Type 2 Diabetes Mellitus Treated with STEGLATRO* and Greater than Placebo in Pooled Placebo-Controlled Clinical Trials (NCT01958671, NCT02033889, and NCT02036515) of STEGLATRO Monotherapy or Combination Therapy
| Number (%) of Patients | |||
|---|---|---|---|
| Placebo N = 515 |
STEGLATRO 5 mg N = 519 |
STEGLATRO 15 mg N = 510 |
|
| Female genital mycotic infections† | 3.0% | 9.1% | 12.2% |
| Male genital mycotic infections‡ | 0.4% | 3.7% | 4.2% |
| Urinary tract infections§ | 3.9% | 4.0% | 4.1% |
| Headache | 2.3% | 3.5% | 2.9% |
| Vaginal pruritus¶ | 0.4% | 2.8% | 2.4% |
| Increased urination# | 1.0% | 2.7% | 2.4% |
| Nasopharyngitis | 2.3% | 2.5% | 2.0% |
| Back pain | 2.3% | 1.7% | 2.5% |
| Weight decreased | 1.0% | 1.2% | 2.4% |
| ThirstÞ | 0.6% | 2.7% | 1.4% |
* The three placebo controlled studies included one monotherapy trial and two add-on combination trials with metformin or with metformin and sitagliptin.
† Includes: genital candidiasis, genital infection fungal, vaginal infection, vulvitis, vulvovaginal candidiasis, vulvovaginal mycotic infection, and vulvovaginitis. Percentages calculated with the number of female patients in each group as denominator: placebo (N=235), STEGLATRO 5 mg (N=252), STEGLATRO 15 mg (N=245).
‡ Includes: balanitis candida, balanoposthitis, genital infection, and genital infection fungal. Percentages calculated with the number of male patients in each group as denominator: placebo (N=280), STEGLATRO 5 mg (N=267), STEGLATRO 15 mg (N=265).
§ Includes: cystitis, dysuria, streptococcal urinary tract infection, urethritis, urinary tract infection.
¶ Includes: vulvovaginal pruritus and pruritus genital. Percentages calculated with the number of female patients in each group as denominator: placebo (N=235), ertugliflozin 5 mg (N=252), ertugliflozin 15 mg (N=245).
# Includes: pollakiuria, micturition urgency, polyuria, urine output increased, and nocturia.
Þ Includes: thirst, dry mouth, polydipsia, and dry throat.
STEGLATRO Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: More genital yeast infections were seen in women taking STEGLATRO compared to men taking STEGLATRO. In the trial, all patients (men and women) treated with STEGLATROI had more genital yeast infections compared to patients given placebo.
- Race: The majority of patients were White. The number of patients of other races was limited; therefore, differences in side effects among races could not be determined.
- Age: Patients above 65 years of age had more side effects related to dehydration compared to younger patients.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the most common adverse reaction, genital mycotic infection, by sex, age, race subgroups based on the safety population of a pool of 3 placebo-controlled trials.
Table 8. Subgroup Analysis of Genital Mycotic Infections (Trials NCT01958671, NCT02033889, and NCT02036515)
| Subgroup | Placebo (N=515) |
STEGLATRO (N=1029) |
||
|---|---|---|---|---|
| n (%) | Total (n) | n (%) | Total (n) | |
| Overall | 8 (1.6) | 515 | 74 (7.2) | 1029 |
| Sex Men Women |
1 (0.4) 7 (3.0) |
280 235 |
21 (3.9) 53 (10.7) |
532 497 |
| Age Group 17 - 64 years ≥65 years |
7 (1.8) 1 (0.8) |
395 120 |
60 (7.6) 14 (6.0) |
794 235 |
| Race White Black or African American Asian American Indian or Alaska Native Native Hawaiian Or other Pacific Islander Other |
8 (2.1) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) |
378 31 79 6 0 21 |
62 (8.2) 3 (4.2) 6 (3.9) 0 (0.0) 0 (0.0) 3 (7.1) |
756 71 154 6 0 42 |
Clinical Trial Data
Patients 65 years and older had a higher incidence of adverse reactions related to volume depletion compared to younger patients; events were reported in 1.1%, 2.2%, and 2.6% of patients treated with comparator, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively.
STEGLATRO Prescribing Information
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved STEGLATRO based on evidence from seven clinical trials (NCT01958671, NCT02033889, NCT02036515, NCT01986855, NCT01999218, NCT02099110, and NCT02226003) of 4,859 patients with type 2 diabetes. Trials were conducted at 387 centers in Asia, Australia/New Zealand, Europe, North America, South Africa, and South America.
Five of the trials (NCT02033889, NCT02036515, NCT01999218, NCT02099110, and NCT02226003) provided support for approval of two new combination drug products (Segluromet and Steglujan) that contain STEGLATRO in combination with the approved antidiabetic drugs metformin and sitagliptin, respectively.
Presented below is the population of 4,859 patients with type 2 diabetes that participated in all 7 trials together. Three of the trials (NCT01958671, NCT02036515, and NCT02033889) were used to evaluate the side effects of STEGLATRO and represent the safety population. Demographics of these patients are presented in Table 10 under MORE INFO section.
Figures 1 – 3 below summarize by sex, race and age how many patients participated in seven combined clinical trials.
Figure 1. Baseline Demographics by Sex
FDA Review
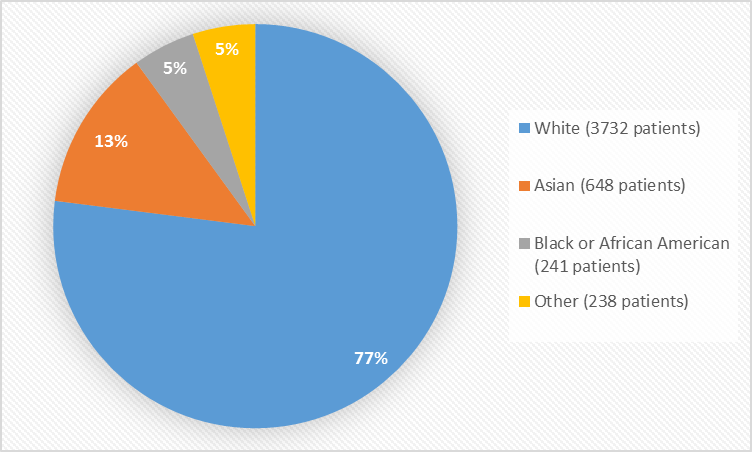
Figure 2 and Table 1 summarize the percentage of patients by race in clinical trials.
Figure 2. Baseline Demographics by Race
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 3732 | 77 |
| Black or African American | 241 | 5 |
| Asian | 648 | 13 |
| American Indian or Alaska Native | 59 | 1 |
| Native Hawaiian or Other Pacific Islander | 2 | less than 1 |
| Multiple | 177 | 4 |
FDA Review
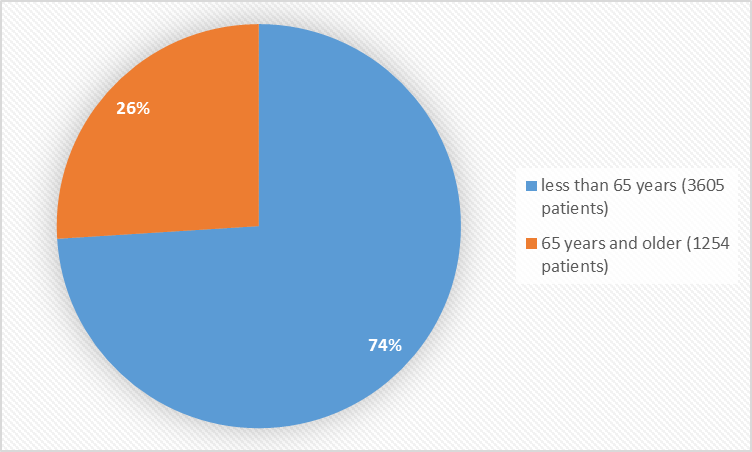
Figure 3 summarizes the percentage of patients by age in clinical trials.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The table below summarizes patients who participated in the 7 trials.
Table 9. Baseline Demographics for Patients in STEGLATRO Trials
| Demographic Parameter | Non-STEGLATRO (N=1450) n (%) |
STEGLATRO (N=3409) n (%) |
Total (N=4859) n (%) |
|---|---|---|---|
| Sex | |||
| Men | 787 (54.3) | 1729 (50.7) | 2516 (51.8) |
| Women | 663 (45.7) | 1680 (49.3) | 2343 (48.2) |
| Age Group (years) | |||
| < 65=""> | 1072 (73.9) | 2533 (74.3) | 3605(74.2) |
| > 65 years | 378 (26.1) | 876 (25.7) | 1254(25.8) |
| Race | |||
| White | 1113 (76.8) | 2619 (76.8) | 3732 (76.8) |
| Black or African American | 75 (5.2) | 166 (4.9) | 241 (5.0) |
| Asian | 190 (13.1) | 458 (13.4) | 648 (13.3) |
| American Indian or Alaska Native | 18 (1.2) | 41 (1.2) | 59 (1.2) |
| Native Hawaiian or Other Pacific Islander | 1 (0.1) | 1 (0.0) | 2 (0.0) |
| Multiple | 53 (3.7) | 124 (3.6) | 177 (3.6) |
| Ethnicity | |||
| Hispanic or Latino | 333 (23.0) | 845 (24.8) | 1178 (24.2) |
| Not Hispanic or Latino | 1114 (76.8) | 2563 (75.2) | 3677 (75.7) |
| Not Reported | 2 (0.1) | 1 (0.0) | 3 (0.1) |
| Unknown | 1 (0.1) | 0 (0.0) | 1 (0.0) |
Clinical Summary Report
The table below summarizes the safety population of patients with type 2 diabetes, who participated in the 3 placebo-controlled trials.
Table 10. Baseline Demographics for Safety Population of Three Placebo-Controlled Clinical Trials (NCT01958671, NCT02033889, and NCT02036515)
| Demographic Parameter | Placebo (N=515) n (%) |
STEGLATRO (N=1029) n (%) |
|
|---|---|---|---|
| Sex | |||
| Men | 280 (54.4) | 532 (52.0) | |
| Women | 235 (45.6) | 497 (48.3) | |
| Age Group (years) | |||
| <65> | 395 (76.7) | 794 (77.2) | |
| >65 years | 120 (23.3) | 235 (22.8) | |
| Race | |||
| White | 378 (73.4) | 756 (73.5) | |
| Black or African American | 31 (6.0) | 71 (6.9) | |
| Asian | 79 (15.3) | 154 (15.0) | |
| American Indian or Alaska Native | 6 (1.2) | 6 (0.6) | |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | 0 (0.0) | |
| Other | 21 (4.1) | 42 (4.1) | |
| Ethnicitya | |||
| Hispanic or Latino | 101 (19.6) | 187 (18.2) | |
| Not Hispanic or Latino | 412 (80.0) | 842 (81.8) | |
a Ethnicity was not reported by 3 patients in the placebo group.
Clinical Summary Report
How were the trials designed?
The benefits and side effects of STEGLATRO were evaluated in seven clinical trials of adults with type 2 diabetes whose disease was not well controlled. All patients were required to follow diet and exercise recommendations, but the trials differed with respect to which other drugs patients were allowed to use for diabetes treatment.
In five trials, patients were randomly assigned to receive either STEGLATRO or placebo by mouth once daily; and, in two trials, they received either STEGLATRO or an active comparator. Neither the patients nor the healthcare providers knew which treatment patients received until after the trial was completed. Treatment was given for 26 weeks for six of the trials, and for 52 weeks for one trial.
The benefit of STEGLATRO was evaluated by the change in HbA1c between the STEGLATRO and the other treatment (either placebo or active comparator) at the end of the treatment period.
How were the trials designed?
The efficacy and safety of STEGLATRO alone and in combination with metformin and/or sitagliptin were evaluated in seven randomized, double-blind, multi-center, controlled Phase 3 clinical trials of adults with inadequately controlled type 2 diabetes.
In six of the trials the primary endpoint was change in HbA1 measured from start of treatment (baseline) up to 26 weeks while maintaining diet and exercise requirement., The seventh trial measured the HbA1c change from baseline up to 52 weeks.
The designs of the seven trials were as follows:
- Randomized, double-blind, placebo-controlled 26-week trial in patients not on any other drug to treat diabetes (NCT01958671)
- Randomized, double-blind, placebo-controlled 26-week trial in patients receiving metformin (NCT02033889)
- Randomized, double-blind, placebo-controlled 26-week trial in patients receiving metformin and sitagliptin (NCT02036515)
- Randomized, double-blind, placebo-controlled 26-week trial in patients with moderate renal impairment primarily receiving background insulin and/or sulfonylurea therapy (NCT01986855)
- Randomized double-blind active-controlled 52-week non-inferiority trial comparing STEGLATRO to glimepiride on background metformin (NCT01999218)
- Randomized, double-blind, active-controlled, 26-week factorial trial in patients receiving background metformin comparing STEGLATRO combined with sitagliptin to sitagliptin alone and to STEGLATRO alone (NCT02099110)
- Randomized, double-blind, placebo-controlled, 26-weeks trial comparing STEGLATRO combined with sitagliptin in patients not on any other drug to treat diabetes (NCT02226003)
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.