Drug Trials Snapshots: QELBREE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the QELBREE Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
QELBREEE (viloxazine)
Kel’ bree
Supernus Pharmaceuticals, Inc.
Approval date: April 2, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
QELBREE is a prescription medicine used to treat attention deficit hyperactivity disorder (ADHD) in children 6 to 17 years of age.
How is this drug used?
QELBREE extended-release capsules are taken orally one time each day. The healthcare provider will determine the dose of QELBREE.
What are the benefits of this drug?
The benefit of QELBREE (100, 200, and 400 mg) was evaluated in three clinical studies, including two in children (ages 6 to 11 years) and one in adolescents (ages 12 to 17 years) with ADHD. In each study, pediatric patients were randomly assigned to receive one of two doses of QELBREE or placebo once daily for 6 to 8 weeks. None of the patients, their parent(s)/caregiver(s), the study sponsor, or the study doctors knew which treatment the patient received during the study. The severity of ADHD symptoms observed at the last week of treatment was significantly greater in patients who received placebo compared with patients who received QELBREE 100 mg, 200 mg, and 400mg.
What are the benefits of this drug?
Table 1 summarizes efficacy results for the evaluated patients in the three clinical trials. The primary endpoint was the change from baseline (reduction) in ADHD Rating Scale-5 (ADHD-RS-5) total score at Week 6. The number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
Table 1: Primary Efficacy Results for Change from Baseline in ADHD-RS-5 Total Score in Pediatric Patients (6 to 17 years) with ADHD (Studies 1, 2, 3)
Source: QELBREE Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: QELBREE worked similarly in male and female patients.
- Race: QELBREE worked similarly in different racial groups.
- Age: QELBREE worked similarly in the age groups studied.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Tables 2, 3, and 4 below summarize the efficacy results by sex, race, and age, respectively, for Study 1. Tables 5, 6, and 7 below summarize the efficacy results by sex, race, and age, respectively, for Study 2. Tables 8, 9, and 10 below summarize the efficacy results by sex, race, and age, respectively, for Study 3. In all subgroup analyses, the primary endpoint was the change from baseline in ADHD-RS-5 total score at Week 6; the analysis population was the intent-to-treat (ITT) population, defined as all randomized subjects who took at least one dose of study medication and had a baseline and at least one post-baseline ADHD-RS-5 assessment.
Table 2. Study 1 – Subgroup Analysis by Sex: ADHD-RS-5 Total Score (ITT)
| Treatment Group | Baseline | LS◊ Mean Change from Baseline | LS◊ Mean Difference from Placebo | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD*) |
N | Mean | SE# | Mean | SE# | |
| Male | |||||||
| Placebo | 97 | 43.4 (7.19) |
87 | -10.6 | 1.48 | -- | -- |
| QELBREE 100 mg/day |
94 | 45.2 (6.77) |
87 | -15.2 | 1.49 | -4.6 | 2.09 |
| QELBREE 200 mg/day |
99 | 44.4 (6.90) |
95 | -18.0 | 1.44 | -7.3 | 2.05 |
| Female | |||||||
| Placebo | 58 | 43.8 (6.85) |
54 | -11.0 | 1.78 | -- | -- |
| QELBREE 100 mg/day |
53 | 44.8 (6.13) |
52 | -18.7 | 1.84 | -7.6 | 2.51 |
| QELBREE 200 mg/day |
59 | 43.4 (6.65) |
51 | -17.4 | 1.77 | -6.4 | 2.48 |
Source: FDA Review
* Standard deviation, # standard error, ◊ least squares
Table 3. Study 1 – Subgroup Analysis by Race: ADHD-RS-5 Total Score (ITT)
| Treatment Group | Baseline | LS◊ Mean Change from Baseline | LS◊ Mean Difference from Placebo | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD*) |
N | Mean | SE# | Mean | SE# | |
| White | |||||||
| Placebo | 77 | 43.0 (7.62) |
73 | -10.6 | 1.64 | -- | -- |
| QELBREE 100 mg/day |
76 | 45.1 (6.46) |
73 | -17.9 | 1.66 | -7.2 | 2.33 |
| QELBREE 200 mg/day |
83 | 44.0 (7.09) |
80 | -18.4 | 1.58 | -7.8 | 2.27 |
| Other races | |||||||
| Placebo | 78 | 44.1 (6.44) |
68 | -11.5 | 1.59 | -- | -- |
| QELBREE 100 mg/day |
71 | 44.9 (6.64) |
66 | -15.2 | 1.62 | -3.7 | 2.24 |
| QELBREE 200 mg/day |
75 | 44.0 (6.52) |
66 | -17.0 | 1.58 | -5.5 | 2.21 |
Source: FDA Review
* Standard deviation, # standard error, ◊ least squares
Table 4. Study 1 – Subgroup Analysis by Age: ADHD-RS-5 Total Score (ITT)
| Treatment Group | Baseline | LS◊ Mean Change from Baseline | LS◊ Mean Difference from Placebo | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD*) |
N | Mean | SE# | Mean | SE# | |
| Age 6-9 | |||||||
| Placebo | 103 | 44.6 (6.22) | 92 | -9.8 | 1.44 | -- | -- |
| QELBREE 100 mg/day |
103 | 45.9 (6.02) | 97 | -16.5 | 1.42 | -6.7 | 2.03 |
| QELBREE 200 mg/day |
107 | 44.3 (6.58) | 96 | -17.5 | 1.38 | -7.7 | 2.01 |
| Age 10-11 | |||||||
| Placebo | 52 | 41.4 (8.11) | 49 | -12.4 | 1.80 | -- | -- |
| QELBREE 100 mg/day |
44 | 43.0 (7.24) | 42 | -16.0 | 1.96 | -3.6 | 2.67 |
| QELBREE 200 mg/day |
51 | 43.3 (7.27) | 50 | -17.5 | 1.81 | -5.1 | 2.56 |
Source: FDA Review
* Standard deviation, # standard error, ◊ least squares
Table 5. Study 2 - Subgroup Analysis by Sex: ADHD-RS-5 Total Score (ITT)
| Treatment Group | Baseline | LS◊ Mean Change from Baseline | LS◊ Mean Difference from Placebo | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD*) |
N | Mean | SE# | Mean | SE# | |
| Female | |||||||
| Placebo | 36 | 44.4 (6.95) |
35 | -13.7 | 2.52 | -- | -- |
| QELBREE 200 mg/day |
33 | 44.8 (6.78) |
31 | -20.3 | 2.67 | -6.6 | 3.63 |
| QELBREE 400 mg/day |
38 | 43.5 (6.45) |
32 | -18.3 | 2.54 | -4.6 | 3.55 |
| Male | |||||||
| Placebo | 61 | 43.0 (6.71) |
57 | -10.8 | 1.85 | -- | -- |
| QELBREE 200 mg/day |
74 | 43.4 (6.42) |
68 | -16.6 | 1.71 | -5.7 | 2.50 |
| QELBREE 400 mg/day |
59 | 46.0 (6.46) |
53 | -16.9 | 1.93 | -6.0 | 2.66 |
Source: FDA Review
* Standard deviation, # standard error, ◊ least squares
Table 6. Study 2 – Subgroup Analysis by Race: ADHD-RS-5 Total Score (ITT)
| Treatment Group | Baseline | LS◊ Mean Change from Baseline | LS◊ Mean Difference from Placebo | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD*) |
N | Mean | SE# | Mean | SE# | |
| White | |||||||
| Placebo | 53 | 43.8 (6.79) |
52 | -13.7 | 2.05 | -- | -- |
| QELBREE 200 mg/day |
54 | 43.9 (6.27) |
51 | -20.1 | 2.07 | -6.4 | 2.90 |
| QELBREE 400 mg/day |
52 | 45.0 (6.65) |
49 | -20.4 | 2.12 | -6.7 | 2.92 |
| Other races | |||||||
| Placebo | 44 | 43.2 (6.86) |
40 | -9.2 | 2.08 | -- | -- |
| QELBREE 200 mg/day |
53 | 43.7 (6.85) |
48 | -15.0 | 1.92 | -5.8 | 2.80 |
| QELBREE 400 mg/day |
45 | 45.0 (6.49) |
36 | -13.9 | 2.12 | -4.7 | 2.95 |
Source: FDA Review
* Standard deviation, # standard error, ◊ least squares
Table 7: Study 2 – Subgroup Analysis by Age: ADHD-RS-5 Total Score (ITT)
| Treatment Group | Baseline | LS◊ Mean Change from Baseline | LS◊ Mean Difference from Placebo | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD*) | N | Mean | SE# | Mean | SE# | |
| Age 6-9 | |||||||
| Placebo | 64 | 44.0 (6.76) | 61 | -11.2 | 1.84 | -- | -- |
| QELBREE 200 mg/day |
71 | 44.3 (6.40) | 64 | -17.4 | 1.79 | -6.2 | 2.56 |
| QELBREE 400 mg/day |
68 | 45.6 (6.29) | 59 | -18.5 | 1.83 | -7.3 | 2.60 |
| Age 10-11 | |||||||
| Placebo | 33 | 42.7 (6.88) | 31 | -11.6 | 2.43 | -- | -- |
| QELBREE 200 mg/day |
36 | 42.8 (6.78) | 35 | -17.5 | 2.32 | -5.9 | 3.36 |
| QELBREE 400 mg/day |
29 | 43.7 (7.04) | 26 | -14.3 | 2.63 | -2.7 | 3.58 |
Source: FDA Review
* Standard deviation, # standard error, ◊ least squares
Table 8. Study 3 – Subgroup Analysis by Sex: ADHD-RS-5 Total Score (ITT)
| Treatment Group | Baseline | LS◊ Mean Change from Baseline | LS◊ Mean Difference from Placebo | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD*) | N | Mean | SE# | Mean | SE# | |
| Male | |||||||
| Placebo | 58 | 40.7 (6.48) | 57 | -8.70 | 1.77 | -- | -- |
| QELBREE 200 mg/day |
66 | 40.2 (7.14) | 60 | -15.8 | 1.66 | -7.0 | 2.39 |
| QELBREE 400 mg/day |
67 | 39.3 (7.77) | 62 | -18.0 | 1.64 | -9.3 | 2.38 |
| Female | |||||||
| Placebo | 46 | 40.3 (7.23) | 45 | -14.7 | 2.15 | -- | -- |
| QELBREE 200 mg/day |
28 | 39.3 (7.52) | 28 | -16.5 | 2.77 | -1.9 | 3.50 |
| QELBREE 400 mg/day |
36 | 39.4 (7.35) | 36 | -13.9 | 2.43 | 0.8 | 3.25 |
Source: FDA Review
* Standard deviation, # standard error, ◊ least squares
Table 9. Study 3 – Subgroup Analysis by Race: ADHD-RS-5 Total Score (ITT)
| Treatment Group | Baseline | LS◊ Mean Change from Baseline | LS◊ Mean Difference from Placebo | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD*) |
N | Mean | SE# | Mean | SE# | |
| White | |||||||
| Placebo | 63 | 40.5 (6.64) |
62 | -12.3 | 1.73 | -- | -- |
| QELBREE 200 mg/day |
53 | 40.3 (7.04) |
53 | -19.7 | 1.87 | -7.3 | 2.54 |
| QELBREE 400 mg/day |
55 | 38.8 (7.41) |
54 | -16.5 | 1.85 | -4.1 | 2.52 |
| Other races | |||||||
| Placebo | 41 | 40.5 (7.11) |
40 | -9.9 | 2.19 | -- | -- |
| QELBREE 200 mg/day |
41 | 39.4 (7.51) |
35 | -10.3 | 2.28 | -0.4 | 3.10 |
| QELBREE 400 mg/day |
48 | 40.0 (7.82) |
44 | -16.3 | 2.05 | -6.4 | 2.96 |
Source: FDA Review
* Standard deviation, # standard error, ◊ least squares
Table 10. Study 3 – Subgroup Analysis by Age: ADHD-RS-5 Total Score (ITT)
| Treatment Group | Baseline | LS◊ Mean Change from Baseline | LS◊ Mean Difference from Placebo | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD*) | N | Mean | SE# | Mean | SE# | |
| Age 12-14 | |||||||
| Placebo | 70 | 41.6 (6.36) | 68 | -9.7 | 1.62 | -- | -- |
| QELBREE 200 mg/day |
63 | 41.0 (6.80) | 58 | -16.9 | 1.73 | -7.1 | 2.37 |
| QELBREE 400 mg/day |
64 | 40.3 (7.37) | 60 | -16.4 | 1.71 | -6.7 | 2.36 |
| Age 15-17 | |||||||
| Placebo | 34 | 38.3 (7.21) | 34 | -15.1 | 2.44 | -- | -- |
| QELBREE 200 mg/day |
31 | 37.8 (7.70) | 30 | -14.4 | 2.57 | 0.7 | 3.55 |
| QELBREE 400 mg/day |
39 | 37.8 (7.77) | 38 | -16.7 | 2.30 | -1.7 | 3.35 |
Source: FDA Review
* Standard deviation, # standard error, ◊ least squares
What are the possible side effects?
The most common side effects of QELBREE are sleepiness, decreased appetite, tiredness, nausea, vomiting, trouble sleeping, and irritability. QELBREE can increase the risk of suicidal thoughts or actions. Other possible side effects of QELBREE include increased blood pressure and heart rate, manic episodes, and effects on weight.
What are the possible side effects (results of trials used to assess safety)?
Table 11: Adverse Reactions Reported in ≥2% of Pediatric Patients (6 to 17 Years of Age) Treated with QELBREE and at a Rate Greater than Placebo-Treated Patients in Placebo-Controlled ADHD Studies
| Body System Adverse Reaction | Placebo N=463 (%) |
QELBREE |
|||
|---|---|---|---|---|---|
| 100mg N=154 (%) |
200mg N=367 (%) |
400mg N=305 (%) |
All QELBREE N=826 (%) |
||
| Nervous system disorders | |||||
| Somnolence* | 4 | 12 | 16 | 19 | 16 |
| Headache* | 7 | 10 | 11 | 11 | 11 |
| Metabolic and nutritional disorders | |||||
| Decreased appetite | 0.4 | 5 | 8 | 8 | 7 |
| Infections and infestations | |||||
| Upper respiratory tract infection* | 6 | 5 | 7 | 8 | 7 |
| Body as a Whole – General disorders | |||||
| Fatigue | 2 | 4 | 5 | 9 | 6 |
| Pyrexia | 0.2 | 3 | 2 | 1 | 2 |
| Gastrointestinal system disorders | |||||
| Abdominal Pain* | 4 | 3 | 6 | 7 | 5 |
| Nausea | 3 | 1 | 4 | 7 | 5 |
| Vomiting | 2 | 5 | 3 | 6 | 4 |
| Psychiatric disorders | |||||
| Insomnia* | 1 | 2 | 5 | 5 | 4 |
| Irritability | 1 | 3 | 2 | 5 | 3 |
Source: QELBREE Prescribing Information
*The following terms were combined:
Somnolence: somnolence, lethargy, sedation
Headache: headache, migraine, migraine with aura, tension headache
Upper respiratory tract infection: nasopharyngitis, pharyngitis, sinusitis, upper respiratory tract infection, viral sinusitis, viral upper respiratory tract infection
Abdominal pain: abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper
Insomnia: initial insomnia, insomnia, middle insomnia, poor quality sleep, sleep disorder, terminal insomnia
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in male and female patients.
- Race: The occurrence of side effects was similar in different racial groups.
- Age: The occurrence of side effects was similar in patients ages 6 to 11 years and patients ages 12 to 17 years.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 12: Adverse Events by Demographic Subgroup, Safety Population
| Characteristic | Treatment N=826 [n/Ns (%)] |
Placebo N=463 [n/Ns (%)] |
|---|---|---|
| Sex, n (%) | ||
| Male | 282/539 (52.3) | 93/285 (32.6) |
| Female | 140/287 (48.7) | 78/178 (43.8) |
| Age group, years, n (%) | ||
| 6-9 | 187/356 (52.5) | 59/175 (33.7) |
| 10-11 | 78/166 (47) | 35/87 (40.2) |
| 12-14 | 99/193 (51.2) | 49/134 (36.6) |
| 15-17 | 58/111 (52.2) | 28/67 (41.8) |
| Race, n (%) | ||
| American Indian or Alaska Native | 5/5 (100) | 4/4 (100) |
| Black or African American | 165/340 (48.5) | 51/175 (29.1) |
| Multiple | 19/31 (61.2) | 9/19 (47.4) |
| White | 231/448 (51.6) | 105/263 (39.9) |
| Asian | 2/2 (100) | 2/2 (100) |
Source: FDA Review
Abbreviations: N, number of subjects in treatment group; n, number of subjects with given characteristic; Ns, number of subjects in subgroup
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved QELBREE based on evidence from several clinical trial(s) of 1289 patients with attention deficit hyperactivity disorder (ADHD). The trials were conducted at 59 of sites in the United States.
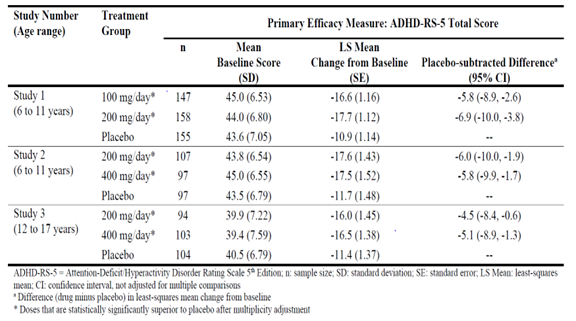
Figure 1 summarizes how many males and females were enrolled in the three combined clinical trials used to evaluate the efficacy of QELBREE.
Figure 1: Baseline Demographics by Sex – Efficacy Population
Source: FDA Review
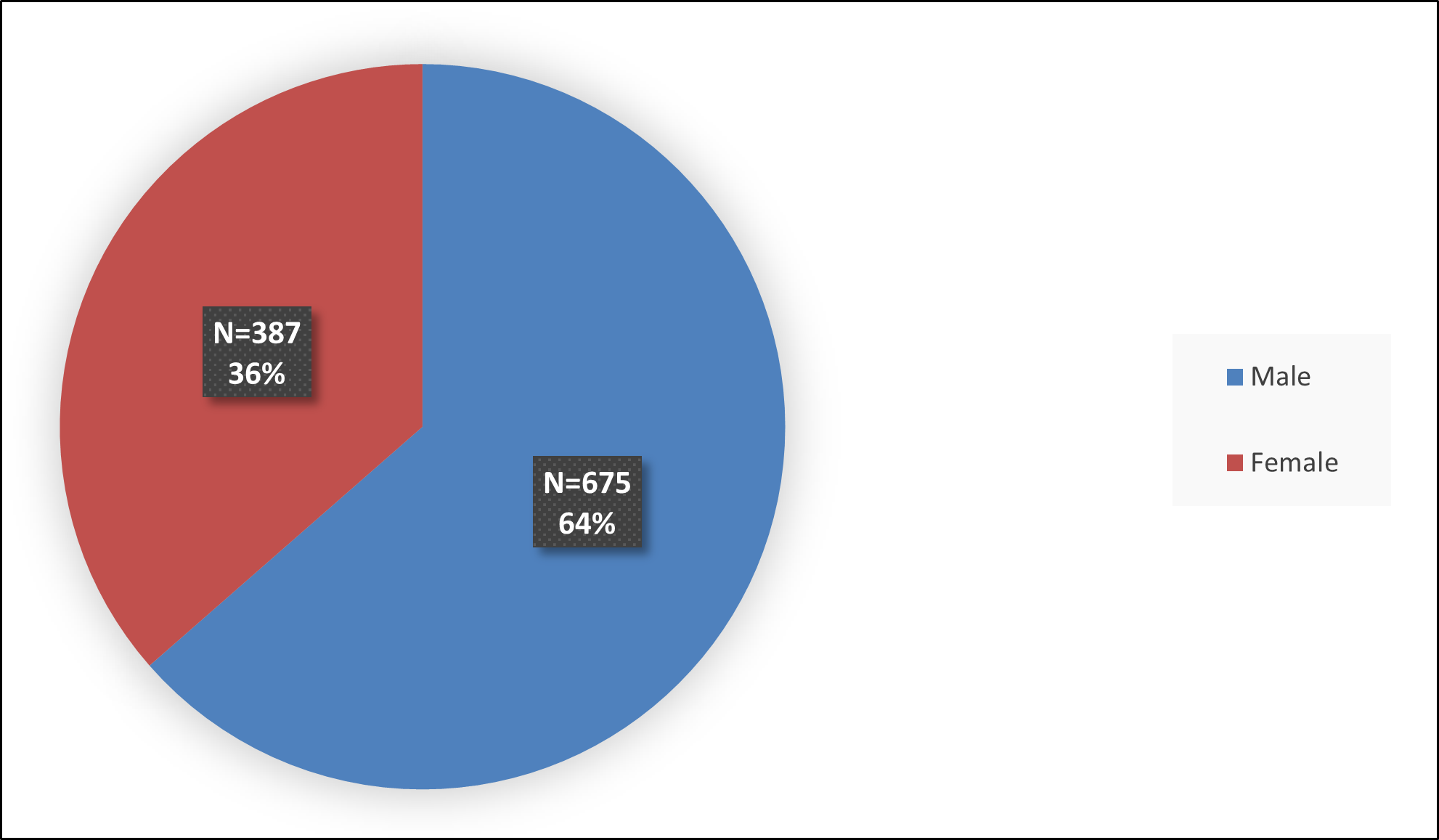
Figure 2 below summarizes how many patients by sex were in the combined trials (Studies 1, 2, and 3, and a PK study) used to evaluate the side effects of QELBREE.
Figure 2: Baseline Demographics by Sex – Safety Population
Source: FDA Review
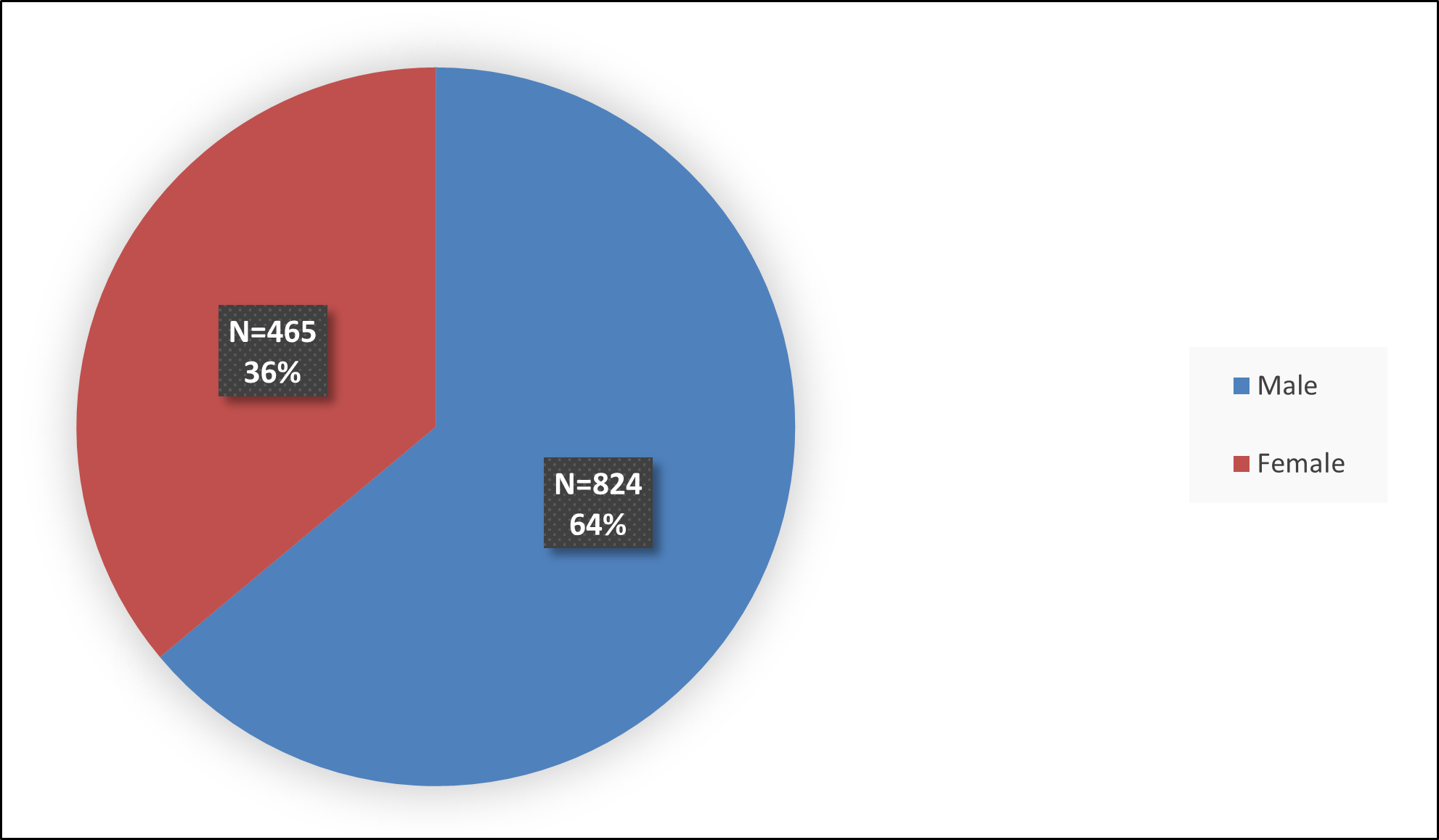
Figure 3 summarizes the percentage of patients by race enrolled in the three combined clinical trials used to evaluate the efficacy of QELBREE.
Figure 3: Baseline Demographics by Race – Efficacy Population
Source: FDA Review
Figure 4 summarizes how many patients by race were in the combined trials (Studies 1, 2, and 3, and a PK study) used to evaluate the side effects of QELBREE.
Figure 4: Demographics of Safety Trials by Race
Source: FDA Review
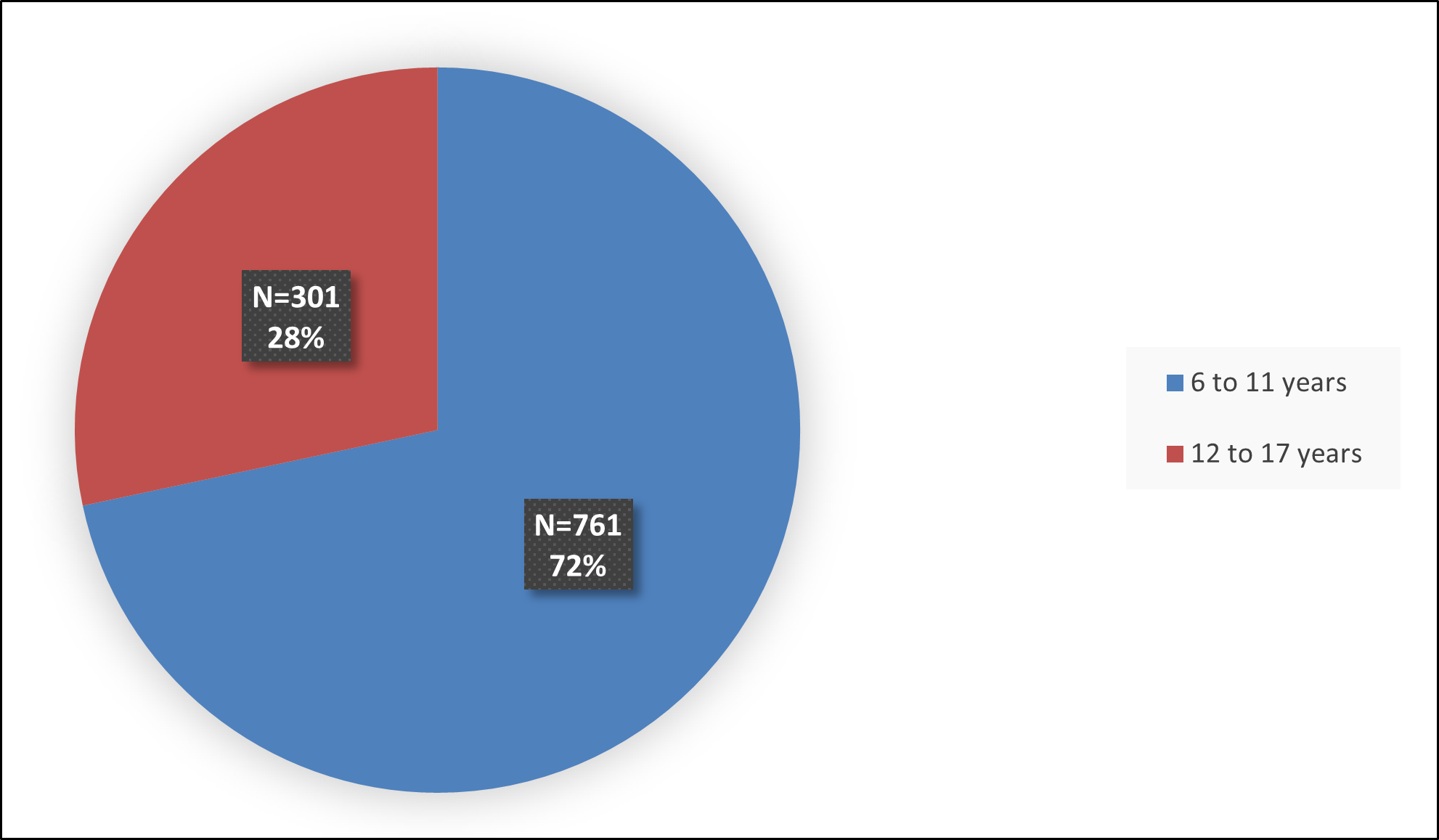
Figure 5 summarizes the percentage of patients by age enrolled in the three combined clinical trials used to evaluate the efficacy of QELBREE.
Figure 5: Baseline Demographics by Age (in Years) – Efficacy Population
Source: FDA Review
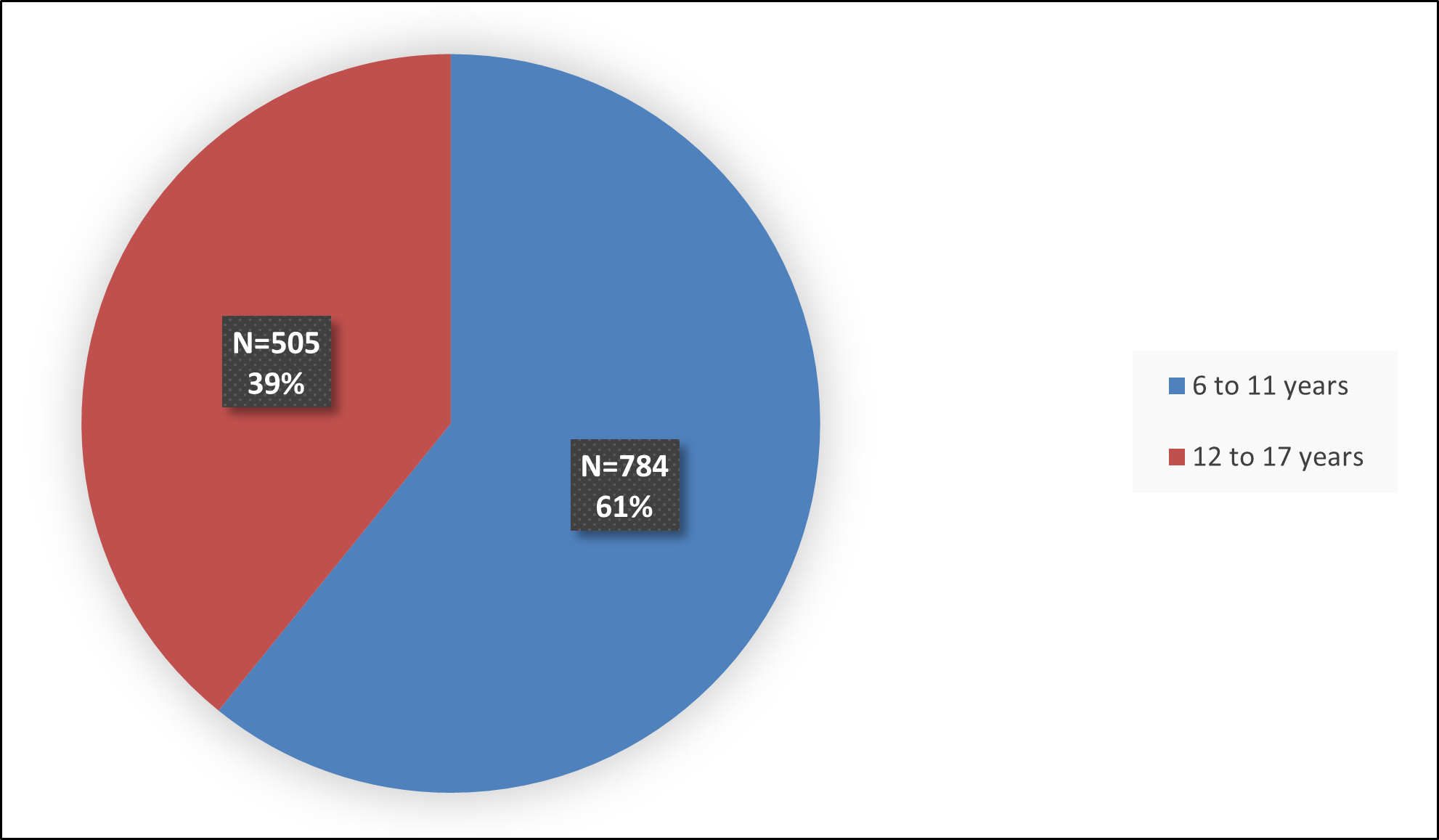
Figure 6 summarizes how many patients by age were in the combined trials (Studies 1, 2, and 3, and a PK study) used to evaluate the side effects of QELBREE.
Figure 6: Baseline Demographics by Age (in Years) – Safety Population
Source: FDA Review
Who participated in the trials?
Table 13: Baseline Demographic Characteristics, Safety Population
| Ages 6 to 11 Years | Ages 12 to 17 Years | Total | ||||
|---|---|---|---|---|---|---|
| Characteristic | Treatment N=522 |
Placebo N=262 |
Treatment N=304 |
Placebo N=201 |
Treatment N=826 |
Placebo N=463 |
| Sex, n (%) | ||||||
| Male | 335 (64) | 166 (63) | 204 (67) | 119 (59) | 611 (66) | 285 (62) |
| Female | 187 (36) | 96 (37) | 100 (33) | 82 (41) | 314 (34) | 178 (38) |
| Age group, years, n (%) | ||||||
| 6–9 | 356 (68) | 175 (67) | 0 | 0 | 356 (39) | 175 (38) |
| 10–11 | 166 (32) | 87 (33) | 0 | 0 | 166 (18) | 87 (19) |
| 12–14 | 0 | 0 | 193 (63) | 134 (67) | 267 (29) | 134 (29) |
| 15–17 | 0 | 0 | 111 (37) | 67 (33) | 136 (15) | 67 (15) |
| Race, n (%) | ||||||
| American Indian or Alaska Native | 2 (0.4) | 3 (1) | 3 (1) | 1 (1) | 6 (1) | 4 (1) |
| Black or African American | 226 (43) | 111 (42) | 114 (38) | 64 (32) | 368 (40) | 175 (38) |
| Multiple | 22 (4) | 11 (4) | 9 (3) | 8 (4) | 33 (4) | 19 (4) |
| White | 272 (52) | 135 (52) | 176 (58) | 128 (64) | 515 (56) | 263 (57) |
| Asian | 0 | 2 (1) | 2 (<1) | 0 | 2 (0.2) | 2 (0.4) |
Source: FDA Review
Includes participants enrolled in the Study 1, 2, and 3 clinical trials and a 4th pharmacokinetic (PK) trial in healthy volunteers treated with approved doses of QELBREE
How were the trials designed?
QELBREE was evaluated in four clinical trials of patients with attention deficit hyperactivity disorder (ADHD).
Three studies were conducted to assess the benefit and safety of QELBREE in children and adolescents (6 to 17 years of age) with attention deficit hyperactivity disorder (ADHD). The severity of ADHD symptoms was assessed using the Attention-Deficit Hyperactivity Disorder Rating Scale 5th Edition (ADHD-RS-5) before treatment (baseline) and every week during treatment, including the final week (endpoint). A fourth study provided information about the safety of QELBREE in adolescents 12 to 17 years of age with ADHD.
How were the trials designed?
In Study 1, the benefit of QELBREE was evaluated in children 6 to 11 years of age with ADHD. A total of 477 pediatric patients were randomly assigned to receive one of three treatments (100 mg QELBREE, 200 mg QELBREE, or placebo) once a day for 6 weeks. The overall improvement of ADHD symptoms observed at Week 6 of treatment was significantly greater in children who received either 100 mg or 200 mg QELBREE compared with children who received placebo.
In Study 2, the benefit of QELBREE was evaluated in children 6 to 11 years of age with ADHD. A total of 313 children were randomly assigned to receive one of three treatments (200 mg QELBREE, 400 mg QELBREE, or placebo) once a day for 8 weeks. The overall improvement of ADHD symptoms observed at Week 8 of treatment was significantly greater in children who received either 200 mg or 400 mg QELBREE compared with children who received placebo.
In Study 3, the benefit of QELBREE was evaluated in adolescents 12 to 17 years of age with ADHD. A total of 310 adolescent patients were randomly assigned to receive one of three treatments (200 mg QELBREE, 400 mg QELBREE, or placebo) once a day for 6 weeks. The overall improvements of ADHD symptoms observed at Week 6 of treatment was significantly greater in adolescents who received either 200 mg or 400 mg QELBREE compared with adolescents who received placebo.
In addition to Studies 1, 2, and 3, a fourth study provided information about the safety of QELBREE in adolescents 12 to 17 years of age with ADHD. In these four studies, a total of 826 patients 6 to 17 years of age received treatment with QELBREE and 463 patients received placebo. The most common side effects were sleepiness, decreased appetite, tiredness, nausea, vomiting, trouble sleeping, and irritability. Other important side effects included an increased risk of suicidal ideation and behavior, increases in blood pressure and heart rate, and effects on weight.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION