Drug Trials Snapshots: PRAXBIND
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the PRAXBIND Prescribing Information for complete information.
PRAXBIND (idarucizumab)
Prax-bind
Boehringer-Ingelheim
Approval date: October 16, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
PRAXBIND is a drug to be used during emergency or life-threatening situations when there is a need to reverse the blood-thinning effects of Pradaxa, an anticoagulant, commonly called a “blood thinner”.
How is this drug used?
PRAXBIND is given by intravenous injection.
What are the benefits of this drug?
In an ongoing clinical trial of PRAXBIND, the blood-thinning effect of Pradaxa was fully reversed in 89 percent of patients within four hours of receiving PRAXBIND.
What are the benefits of this drug (results of trials used to assess efficacy)?
PRAXBIND is a humanized monoclonal antibody fragment designed to bind to dabigatran and reverse its action as an anticoagulant. It is the first reversal agent for a new class of oral anticoagulants that inhibit the clotting process but require no monitoring with blood tests. PRAXBIND is specific for Pradaxa; it does not reverse the action of other anticoagulants. Randomized, double-blind, placebo-controlled trials in 283 healthy volunteers explored the safety, effectiveness, dosing, duration of reversal, anti-drug antibodies, and any differences in between women and men, between younger and older subjects, and between subjects with normal or impaired kidney function. These trials showed that
- PRAXBIND infusion of 5 grams in two divided 2.5 gram doses 15 minutes apart resulted in immediate and complete reversal of anticoagulation that was sustained for at least 24 hours. Doses between 1 gram and 4 grams were less effective.
- PRAXBIND infusion resulted in decrease of blood dabigatran to unmeasurable levels by the time infusion of both 2.5 gram doses was completed.
- PRAXBIND activity was the same in women (N=19) as in men (N=98), in Asians (N=37) as in Whites (N=79), in patients less than 65 years of age (N=87) and in patients older than 65 (N=30), and in subjects with mildly impaired kidney function (N=19) as in those with normal kidney function (N=97).
- PRAXBIND is mostly (99%) excreted by the kidneys in urine either unchanged or broken down.
The clinical trial is a multicenter, international, open label safety and efficacy trial with projected enrollment of up to 500 adult patients who were on treatment with Pradaxa and require, in the opinion of the physician, a reversal agent for life-threatening or uncontrolled bleeding or who require emergency surgery or urgent procedures. Interim findings are available on 123 patients; 66 presented with uncontrolled or life-threatening bleeding and 57 required urgent surgery. The patients were 48 to 93 years of age (median age was 77), 40% had moderate or severe insufficiency of kidney function, 95% had atrial fibrillation as reason for treatment with Pradaxa. Among patients who presented with bleeding, 41% had bleeding from stomach or bowels and 36% bleeding in or around the brain. The most common conditions requiring urgent surgery were bone fractures (23%).
A complete normalization of coagulation tests was achieved in 89% of patients within 10 – 30 minutes after PRAXBIND infusion and was sustained for 24 hours. There were a few patients who required one or two additional doses of PRAXBIND to maintain normal coagulation tests. Bleeding stopped within 72 hours in most bleeding patients (the median time was 9.8 hours). Among patients needing emergency surgery, most had normal bleeding during surgery (92%), a few mildly increased bleeding (6%) and one patient had moderately increased but controllable bleeding. Pradaxa was restarted in 26% of bleeding patients and 60% of urgent surgery patients.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: PRAXBIND worked similarly in men and women.
- Race: Most patients in the trial were white. PRAXBIND worked similarly in all races studied.
- Age: Most patients in the trial were 65 years and above. PRAXBIND worked similarly among all ages studied.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The overall median response was 100% for both endpoints. Therefore, response by subgroups is not shown.
Table 2. Overall summary of maximum reversal (%) for ECT from central lab for subgroups (using 100% ULN) in treated patients with PD measurement
| Median Maximum Reversal (95% CI) | n | |
Overall Response | 100% (100.0,100.0) | 68 |
Clinical Trial Data
Table 3. Overall summary of maximum reversal (%) for dTT* from central lab for subgroups (using 100% ULN) in treated patients with PD measurement
| Median Maximum Reversal (95% CI) | n | |
Overall Response | 100% (100.0,100.0) | 68 |
*dTT is not approved or commercially available in the United States
Clinical Trial Data
What are the possible side effects?
In patients requiring surgery or having life-threatening or uncontrolled bleeding, the most common side effects are low potassium (hypokalemia), confusion, constipation, fever, and pneumonia.
Reversing the effect of dabigatran with PRAXBIND also exposes patients to the risk of blood clots and stroke from their underlying disease.
What are the possible side effects (results of trials used to assess safety)?
In the ongoing clinical trial of patients who are taking Pradaxa and require reversal of anticoagulation with PRAXBIND, the most common side effects were low potassium (hypokalemia), confusion, constipation, fever and pneumonia. Since the clinical trial is a case series of patients with present with bleeding or require urgent surgery, these side effects can be due to their medical conditions, complications of these conditions, other medications, as well as to PRAXBIND.
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The number of patients in the main trial was small. Differences between men and women could not be determined.
- Race: Most patients in the trial were white. Differences among races could not be determined.
- Age: Most patients in the trial were 65 years and above. Differences between patients below and above 65 years could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Overall, the trial population was not large enough to conclude whether there were differences in side effects among sex, race, and age groups.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved PRAXBIND based on evidence from three trials in healthy volunteers and one ongoing trial in patients who were taking Pradaxa and were treated with PRAXBIND because they have life-threatening or uncontrolled bleeding, or who need emergency surgery or an urgent procedure. At the time of approval of PRAXBIND, 123 patients had been evaluated in the ongoing patient trial.
Figure 1 summarizes how many men and women are enrolled in the ongoing clinical trial.
Figure 1. Baseline Demographics by Sex
Clinical Trial Data
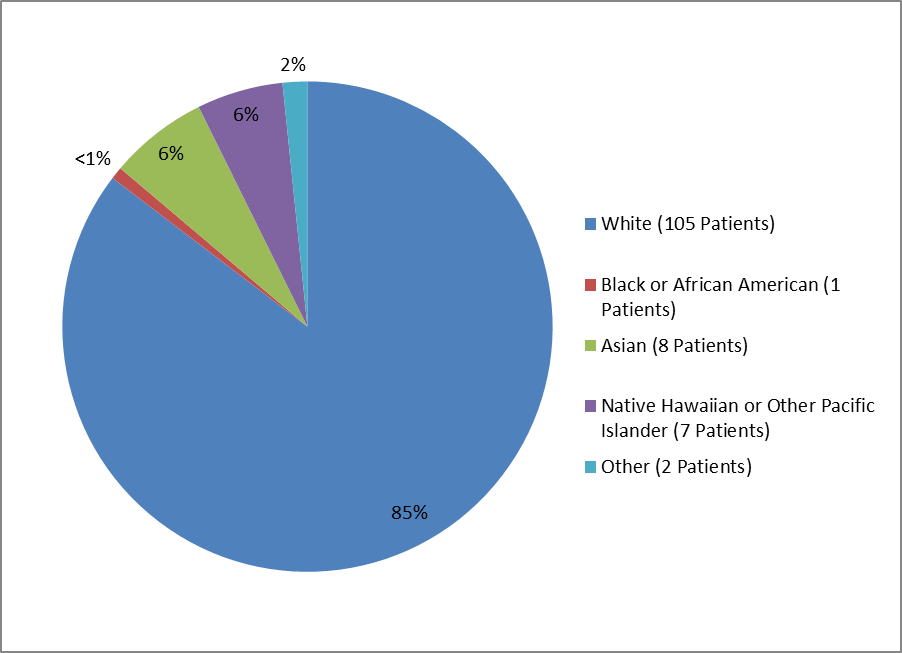
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race
1%=less than="">
Clinical Trial Data
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 105 | 82% |
| Black or African American | 1 | > |
| Asian | 8 | 6% |
| Native Hawaiian or Other Pacific Islander | 7 | 5% |
| Other | 2 | 2% |
Clinical Trial Data
Figure 3 summarizes the percentage of patients by age group enrolled in the clinical trial.
Figure 3. Baseline Demographics by Age
Clinical Trial Data
Who participated in the trials?
The table below summarizes the baseline demographics for 123 patients enrolled in the clinical trial of patients taking dabigatran (PRADAXA) who required PRAXBIND because of uncontrolled bleeding or because they required emergency surgery.
Table 4. Baseline Demographics for Ongoing Single Cohort Case Series Trial
| Demographic Parameters | N=123 n (%) |
|---|---|
| Sex | |
| Men | 65 (53%) |
| Women | 58 (47%) |
| Age | |
| Mean years (SD) | 76.5 |
| Median (years) | 77 |
| Min, Max (years) | 48,93 |
| Age Group | |
| below 65 years | 12 (10%) |
| 65 and above | 111 (90%) |
| Race | |
| White | 105 (85%) |
| Black or African American | 1 (1%) |
| Asian | 8 (7%) |
| American Indian or Alaska Native | 0 (0) |
| Native Hawaiian or Other Pacific Islander | 7 (6%) |
| Other | 2 (2%) |
| Region | |
| Asia | 8 (7%) |
| Europe | 64 (52%) |
| North America | 11 (9%) |
| Other | 40 (32) |
Clinical Trial Data
How were the trials designed?
The three trials of healthy volunteers enrolled people who did not require a blood thinner. These volunteers were given Pradaxa (generic name dabigatran etexilate) and then various amounts of PRAXBIND to establish the effective dose and note side effects. The amount of dabigatran in their blood and the extent of anticoagulation (how much their blood was “thinned”) were measured before and at various times after PRAXBIND was given.
In the ongoing trial of patients who are taking Pradaxa because they require a blood thinner for a medical condition, PRAXBIND was given because of uncontrolled bleeding or because they required emergency surgery. The trial measures how well PRAXBIND can reverse the blood-thinning effect of Pradaxa in patients.
How were the trials designed?
Three randomized, placebo-controlled trials in a total of 283 healthy volunteers assessed the safety, dose-response, and effect of PRAXBIND on reducing unbound dabigatran and normalizing coagulation parameters.
In an ongoing single cohort case series trial, 5 g PRAXBIND was administered to patients treated with dabigatran who presented with dabigatran-related life-threatening or uncontrolled bleeding or who required emergency surgery or urgent procedures.
In this trial, the primary endpoint is the maximum percentage reversal of the anticoagulant effect of dabigatran within 4 hours after the administration of PRAXBIND, based on central laboratory determination of dTT or ECT.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION