Drug Trials Snapshots: PIQRAY
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the PIQRAY Package Insert for complete information.
PIQRAY (alpelisib)
pik’ raye
Novartis Pharmaceuticals Corporation
Approval date: May 24, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
PIQRAY is a drug for treatment of adults with a specific form of advanced breast cancer that progressed or recurred after previous hormone therapy. It is to be used in patients with:
- hormone receptor (HR) positive cancer,
- human epidermal growth factor receptor 2 (HER2)-negative cancer,
- an abnormal inherited PIK3CA gene, and
- whose cancer has spread to other parts of the body (locally advanced or metastatic)
How is this drug used?
PIQRAY is taken as two tablets once daily with food in combination with fulvestrant (another drug used to treat breast cancer).
What are the benefits of this drug?
In patients with an abnormal inherited PIK3CA gene, those who received PIQRAY combined with fulvestrant experienced a longer time period (11 months) that tumors did not grow after treatment when compared to patients who received fulvestrant combined with placebo (5.7 months).
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 shows efficacy results for PFS for patients after treatment with PIQRAY used in combination with fulvestrant.
Table 2. Efficacy Results in Patients with a PIK3CA Mutation per Investigator Assessment
| PIQRAY plus fulvestrant | Placebo plus fulvestrant | |
|---|---|---|
| Progression-free survival (PFS) | N = 169 | N = 172 |
| Number of PFS events – n (%) | 103 (61) | 129 (75) |
| Median PFS months (95% CI) | 11.0 (7.5, 14.5) | 5.7 (3.7, 7.4) |
| Hazard ratio (95% CI) | 0.65 (0.50, 0.85) | |
| p-value1 | 0.0013 | |
| Overall Response Rate | N = 126 | N = 136 |
| ORR2 (95% CI) | 35.7 (27.4, 44.7) | 16.2 (10.4, 23.5) |
1 Both log-rank test and Cox proportional hazards model are stratified by prior CDK4/6 inhibitor usage and presence of lung/liver metastases. P-value was compared to prespecified Haybittle-Peto stopping boundary (two-sided p ≤ 0.0398).
2 ORR = percentage of patients with confirmed Complete Response or Partial Response with measurable disease at baseline
No PFS benefit was observed in patients whose tumors did not have a PIK3CA tissue mutation (HR 0.85; 95% CI: 0.58, 1.25).
PIQRAY Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The majority of patients were women. The number of men was limited; therefore, differences in how PIQRAY worked among sex could not be determined.
- Race: The majority of patients were White. The PIQRAY worked similarly among White and Asian patients. The number of patients in other races was limited; therefore, differences in how PIQRAY worked among other races could not be determined.
- Age: PIQRAY works similarly among patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The efficacy results by demographic subgroups based on PFS for the PIK3CA Mutant Cohort are presented below.
Table 3. PFS per Investigator Assessment in Different Subgroups (PIK3CA Mutant Cohort)
| PIQRAY plus fulvestrant n/N (%) |
PIacebo plus fulvestrant n/N (%) |
HR | 95% CI | |
|---|---|---|---|---|
| Race | ||||
| White | 69/117 (59.0) | 80/109 (73.4) | 0.56 | 0.41, 0.78 |
| Asian | 21/34 (61.8) | 3/40 (77.5) | 0.76 | 0.43, 1.35 |
| Other | 12/17 (70.6) | 17/20 (85.0) | 0.91 | 0.41, 2.00 |
| Age | ||||
| < 65 Years Old | 58/95 (61.1) | 64/89 (71.9) | 0.62 | 0.43, 0.89 |
| >= 65 Years Old | 45/74 (60.8) | 65/83 (78.3) | 0.70 | 0.47, 1.03 |
HR=hazard ratio; CI-confidence interval
FDA Review
What are the possible side effects?
PIQRAY may cause serious side effects including:
- severe allergic reactions
- severe skin reactions
- high blood sugar levels
- lung inflammation
- diarrhea
- harm to a fetus
The most common side effects of PRIQAY were high blood sugar levels (hyperglycemia), increased blood creatinine level, diarrhea, rash, increased white blood cell count, increased liver enzymes, nausea, and tiredness.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in patients with advanced HR-positive, HER2-negative advanced breast cancer (safety population).
Table 4. Adverse Reactions Occurring in ≥10% and ≥2% Higher than Placebo Arm in All Grades
| PIQRAY plus fulvestrant N = 284 |
Placebo plus fulvestrant N=287 |
|||
|---|---|---|---|---|
| Adverse reactions | All Grades % |
Grade 3-4 % |
All Grades % |
Grade 3-4 % |
| Gastrointestinal disorders | ||||
| Diarrhea | 58 | 7* | 16 | 0.3* |
| Nausea | 45 | 2.5* | 22 | 0.3* |
| Stomatitis1 | 30 | 2.5* | 6 | 0* |
| Vomiting | 27 | 0.7* | 10 | 0.3* |
| Abdominal pain2 | 17 | 1.4* | 11 | 1* |
| Dyspepsia | 11 | 0* | 6 | 0* |
| General disorders and administration site conditions | ||||
| Fatigue3 | 42 | 5* | 29 | 1* |
| Mucosal inflammation | 19 | 2.1* | 1.0 | 0* |
| Edema peripheral | 15 | 0* | 5 | 0.3* |
| Pyrexia | 14 | 0.7 | 4.9 | 0.3* |
| Mucosal dryness4 | 12 | 0.4* | 4.2 | 0* |
| Infections and infestations | ||||
| Urinary tract infection5 | 10 | 0.7* | 5 | 1* |
| Investigations | ||||
| Weight decreased | 27 | 3.9* | 2.1 | 0* |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 36 | 0.7* | 10 | 0.3* |
| Nervous system disorders | ||||
| Dysgeusia6 | 18 | 0.4* | 3.5 | 0* |
| Headache | 18 | 0.7* | 13 | 0* |
| Skin and subcutaneous tissue disorders | ||||
| Rash7 | 52 | 20* | 7 | 0.3* |
| Alopecia | 20 | 0* | 2.4 | 0* |
| Pruritus | 18 | 0.7* | 6 | 0* |
| Dry skin8 | 18 | 0.4* | 3.8 | 0* |
Grading according to CTCAE Version 4.03
1Stomatitis: including stomatitis, aphthous ulcer and mouth ulceration
2Abdominal pain: abdominal pain, abdominal pain upper, abdominal pain lower
3Fatigue: including fatigue, asthenia
4Mucosal dryness: including dry mouth, mucosal dryness, vulvovaginal dryness
5Urinary tract infection: including UTI and single case of urosepsis
6Dysgeusia: including dysgeusia, ageusia, hypogeusia
7Rash: including rash, rash maculo-papular, rash macular, rash generalized, rash papular, rash pruritic
8Dry skin: including dry skin, skin fissures, xerosis, xeroderma
*No Grade 4 adverse reactions were reported
PIQRAY Prescribing Information
Table 5. Laboratory Abnormalities Occurring in ≥10% of Patients
| PIQRAY plus fulvestrant N=284 |
Placebo plus fulvestrant N= 287 |
|||
|---|---|---|---|---|
| Laboratory Abnormality | All grades % |
Grade 3-4 % |
All grades % |
Grade 3-4 % |
| Hematological parameters | ||||
| Lymphocyte count decreased | 52 | 8 | 40 | 4.5* |
| Hemoglobin decreased | 42 | 4.2* | 29 | 1* |
| Activated Partial Thromboplastin Time (aPTT) prolonged | 21 | 0.7* | 16 | 0.3* |
| Platelet count decreased | 14 | 1.1 | 6 | 0* |
| Biochemical parameters | ||||
| Glucose increased1 | 79 | 39 | 34 | 1 |
| Creatinine increased | 67 | 2.8* | 25 | 0.7* |
| Gamma Glutamyl Transferase (GGT) increased | 52 | 11 | 44 | 10 |
| Alanine Aminotransferase (ALT) increased | 44 | 3.5 | 34 | 2.4* |
| Lipase increased | 42 | 7 | 25 | 6 |
| Calcium (corrected) decreased | 27 | 2.1 | 20 | 1.4 |
| Glucose decreased | 26 | 0.4 | 14 | 0* |
| Potassium decreased | 14 | 6 | 2.8 | 0.7* |
| Albumin decreased | 14 | 0* | 8 | 0* |
| Magnesium decreased | 11 | 0.4* | 4.2 | 0* |
1Glucose increase is an expected laboratory abnormality of PI3K inhibition
*No Grade 4 laboratory abnormalities were reported
PIQRAY Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The majority of patients were women. The number of men was limited; therefore, differences in the occurrence of side effects among sex could not be determined.
- Race: The majority of patients were White. The occurrence of side effects among White and Asian patients was similar. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among other races could not be determined.
- Age: There was a higher incidence of Grade 3-4 hyperglycemia (high blood sugar level) in patients older than 65 years of age when compared to patients younger than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the most common adverse reaction, hyperglycemia, by subgroup.
Table 6. Subgroup Analysis of All grade (1-5) Hyperglycemia (safety population)
| Demographic Characteristic | PRIQAY plus fulvestrant 284 (%) |
Placebo plus fulvestrant 287 (%) |
|---|---|---|
| Sex | ||
| Men | 0 (0) | 0 (0) |
| Women | 181 (64) | 28 (9.8) |
| Race | ||
| White | 126 (63.3) | 18 (10.2) |
| Asian | 39 (66.1) | 6 (9.1) |
| Other** | 16 (61.5) | 4 (9.1) |
| Age Group | ||
| < 65 years | 104 (62.3) | 15 (9.8) |
| >65 years | 78 (66.7) | 13 (9.7) |
**Other includes Black or African American, American Indian or Alaska Native, Other, Unknown
FDA Review
Table 7. Subgroup Analysis of Grade 3-4 Hyperglycemia (safety population)
| Demographic Characteristic | PRIQAY plus fulvestrant 284 (%) |
Placebo plus fulvestrant 287 (%) |
|---|---|---|
| Sex | ||
| Men | 0 (0) | 0 (0) |
| Women | 104 (36.7) | 2 (0.7) |
| Race | ||
| White | 74 (37.2) | 1 (0.6) |
| Asian | 19 (32.2) | 0 (0) |
| Other** | 11 (42.3) | 1 (2.3) |
| Age Group | ||
| < 65 years | 53 (31.7) | 1 (0.7) |
| > 65 years | 52 (44.5) | 1 (0.7) |
**Other includes Black or African American, American Indian or Alaska Native, Other, Unknown
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved PIQRAY based on evidence from one clinical trial (NCT02437318) of 571 patients with HR-positive, HER2-negative, advanced or metastatic breast cancer whose disease had progressed or recurred on or after hormone treatment. The trial was conducted in Asia, Australia, Canada, Europe, Latin American, and the United States.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
*Other includes American Indian or Alaska Native and Unknown
FDA Review
Table 1. Demographics of Trial by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 377 | 66% |
| Black or African American | 8 | 1% |
| Asian | 125 | 22% |
| American Indian or Native Alaskan | 5 | 1% |
| Other | 26 | 5% |
| Unknown | 31 | 5% |
FDA Review
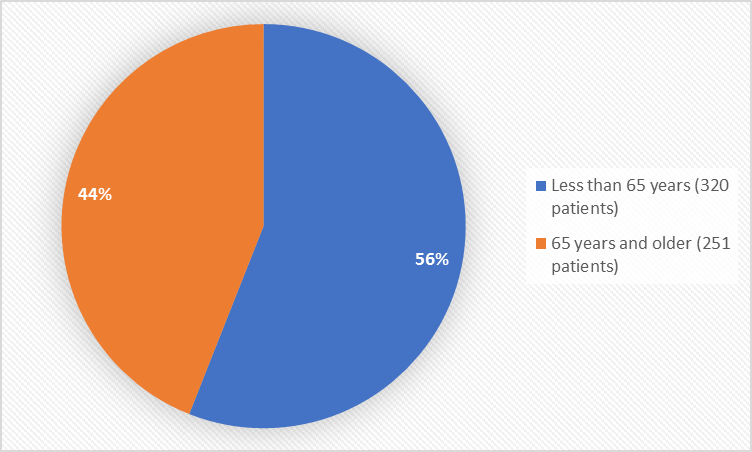
Figure 3 summarizes the percentage of patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The tables below summarizes the demographics of patients in the clinical trial.
Table 8. Demographics of the Safety Population
| PIQRAY + Fulvestrant N=284 n (%) |
Placebo + Fulvestrant N=287 n (%) |
Total N=571* |
|
|---|---|---|---|
| Sex | |||
| Men | 1 (0.4) | 0 (0) | 1 (0.2) |
| Women | 283 (99.6) | 287 (100.0) | 570 (99.8) |
| Race | |||
| White | 199 (70.1) | 177 (61.7) | 376 (65.8) |
| Black or African American | 2 (0.7) | 6 (2.1) | 8 (1.4) |
| Asian | 59 (20.8) | 66 (23.0) | 125 (21.9) |
| American Indian or Alaska Native | 3 (1.1) | 4 (1.4) | 7 (1.2) |
| Other/Unknown | 21 (7.3) | 34 (11.8) | 55 (9.6) |
| Age Group (years) | |||
| < 65 | 167 (58.8) | 153 (53.3) | 320 (56.0) |
| ≥ 65 | 117 (41.2) | 134 (46.7) | 251 (44.0) |
| Age | |||
| Median (Min - Max) years | 62 25-87) | 64 (32-92) | 63 (25-92) |
| Ethnicity | |||
| Hispanic | 40(14.1) | 41 (14.3) | 81 (14.2) |
| Non-Hispanic | 244 (85.9) | 246 (85.7) | 490 (85.8) |
| Region | |||
| Asia | 56 (19.7) | 64 (22.3) | 120 (21.0) |
| Canada | 6 (2.1) | 7 (2.4) | 13 (2.3) |
| Europe | 153 (53.9) | 145 (50.5) | 298 (52.2) |
| Latin America | 17 (6.0) | 26 (9.1) | 43 (7.5) |
| United States | 23 (8.1) | 30 (10.4) | 53 (9.3) |
| Other | 29 (10.2) | 15 (5.2) | 44 (7.7) |
* One patient who was randomized to the placebo plus fulvestrant arm, did not receive treatment because they did not meet the inclusion criteria. This patient was included in the intent to treat (ITT) population (N-172) but was not included in the safety population (N=571).
FDA Review
How were the trials designed?
The benefit and side effects of PIQRAY were evaluated in men and postmenopausal women with advanced HR-positive, HER2-negative breast cancer that had progressed or recurred after treatment with hormone therapy. Some patients had an abnormal PIK3CA gene.
Patients received either PIQRAY or placebo as a tablet by mouth daily in combination with fulvestrant. Fulvestrant was given as an intramuscular injection on Cycle Day 1 and Day 15 of the first 28-day cycle, and then on Cycle Day 1 of every 28-day cycle. The treatment continued until the disease progressed or the side effects became too toxic. Neither the patients nor the health care providers knew which treatment was being given until the trial was completed.
The benefit was assessed by measuring the length of time tumors did not grow after treatment (progression-free survival-PFS).
How were the trials designed?
The efficacy and safety of PIQRAY were evaluated in one clinical trial.
The trial was a randomized, placebo controlled, double blind trial evaluating PIQRAY for the treatment of adult men or postmenopausal women with HR-positive, HER2-negative, advanced or metastatic breast cancer that had progressed or recurred on or after an aromatase inhibitor-based treatment. Patients were enrolled into one of two cohorts (tumor tissue with or without PIK3CA mutation). They were randomized to receive PIQRAY 300 mg or placebo once daily on a continuous basis plus fulvestrant 500 mg administered intramuscularly on Days 1 and 15 of the first 28-day cycle , and then on Day 1 of every subsequent 28-day cycle.
The primary efficacy endpoint was investigator-assessed PFS in the cohort with a PIK3CA mutation per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.