Drug Trials Snapshots: OLINVYK
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the OLINVYK Package Insert for complete information.
OLINVYK (oliceridine)
Oh-LIN-vick
Trevena, Inc.
Approval date: August 7, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
OLINVYK is a drug for the treatment of acute pain in adults when the pain is severe enough to require an intravenous opioid.
How is this drug used?
OLINVYK is an injection. It is administered by a healthcare provider directly into the vein up to 27 milligrams per day.
What are the benefits of this drug?
OLINVYK reduces post-surgical pain. It is not available for home use.
What are the benefits of this drug (results of trials used to assess efficacy)?
The tables below summarize efficacy results for the evaluated patients for Trials 1 and 2. The efficacy was measured using the Summed Pain Intensity Differences (SPID) over 48 hours (SPID-48) or 24 hours (SPID-24), respectively. The SPID is calculated by multiplying the Pain Intensity,
Difference (calculated by subtracting the pain intensity at a particular timepoint from the pain intensity at baseline) scores at each post-baseline timepoint by the duration (in hours) since the preceding timepoint, and then summing the values, over 48 or 24 hours.
Table 1. SPID-48 Efficacy Endpoint Results in Trial 1
|
Efficacy Measure |
|
OLINVYK |
|
|
|
SPID-48 |
|
|
|
|
|
Average |
85 |
138 |
164 |
193 |
|
Differencea |
--- |
47.5* |
80* |
105* |
|
95 % Confidence Interval |
|
(19, 75) |
(52, 108) |
(77, 132) |
Table 2. SPID-24 Efficacy Endpoint Results in Trial 2
|
Efficacy Measure |
OLINVYK |
|
|
||
|
Placebo |
0.35 mg regimen |
0.5 mg regimen |
Morphine |
|
|
|
SPID-24 |
|
|
|
|
|
|
Average |
75 |
90 |
94 |
103 |
|
|
Differencea |
--- |
14* |
18* |
30* |
|
|
95 % Confidence Interval |
|
(2, 26) |
(5, 30) |
(17, 42) |
|
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: OLINVYK worked similarly in men and women.
- Race: OLINVYK worked similarly in White and Black or African American patients. The number of patients of other races was limited; therefore, differences in response for other races could not be determined.
- Age: The number of patients 65 years of age or older was limited; therefore, differences in response between patients younger and older than 65 years could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by subgroups for individual trials.
Table 3. Subgroup Analysis: SPID-48 Efficacy Results from Trial 1
|
Group |
Subgroup |
OLINVYK Difference from Placebo (95% CI)a |
||
|
0.35 mg regimen |
0.5 mg regimen |
|
||
|
Sex |
Women |
48.5 |
83.9 |
|
|
Men |
45.8 |
65.5 |
|
|
|
Raceb |
Black or African American |
37.6 |
80.3 |
|
|
White |
51.8 |
79.4 |
|
|
|
Ageb |
≥ 18 to < 65 years |
45.9 |
82.7 |
|
b Results for patients 65 years or older and for other racial groups are not included due to the low number of enrolled patients in these groups.
Table 4. Subgroup Analysis: SPID-24 Efficacy Results from Trial 2
|
Group |
Subgroup |
OLINVYK Difference from Placebo (95% CI)a |
|
|
|
0.35 mg regimen |
0.5 mg regimen |
|||
|
Sexb |
Women |
14 |
18.1 |
|
|
Raceb |
Black or African American |
17.1 |
20.2 |
|
|
White |
12.2 |
16.1 |
||
|
Ageb |
≥ 18 to < 65 years |
13.8 |
18.9 |
|
b No males were included in either OLINVYK treatment group. c Results for men, patients 65 years or older and other racial groups are not included due to the low number of enrolled patients in these groups
FDA Statistical Review
What are the possible side effects?
OLINVYK may cause serious side effects including:
- addiction and medication abuse,
- life threatening breathing suppression,
- life threatening withdrawal syndrome in a newborn whose mother received OLINVYK during pregnancy,
- breathing problems, deep sedation, coma and death when used with other drugs that suppress brain function (such as benzodiazepines), and
- severe drop in blood pressure.
The most common side effects are trials were nausea, vomiting, dizziness, headache, constipation, itchy skin and low oxygen levels in blood.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions in patients with moderate to severe pain from each trial.
Table 5. Adverse Drug Reactions Reported in ≥5% of OLINVYK -Treated Patients Following Orthopedic Surgery-Bunionectomy (Trial 1)
|
Adverse Drug Reaction |
Placebo |
OLINVYK |
OLINVYK |
Morphineb |
|
(N = 79) |
(N = 79) |
(N = 79) |
(N = 76) |
|
|
Any TEAEc (%) |
68 |
86 |
91 |
96 |
|
Nausea |
24 |
56 |
63 |
65 |
|
Vomiting |
6 |
39 |
41 |
50 |
|
Dizziness |
10 |
32 |
35 |
34 |
|
Somnolence |
6 |
19 |
13 |
13 |
|
Constipation |
11 |
11 |
14 |
17 |
|
Pruritus |
8 |
15 |
4 |
20 |
|
Hypoxia |
0 |
5 |
9 |
9 |
|
Sedation |
1 |
5 |
4 |
3 |
|
Oxygen Saturation Decreased |
0 |
4 |
5 |
9 |
Table 6. Adverse Drug Reactions Reported in ≥5% of OLINVYK -Treated Patients Following Plastic Surgery-Abdominoplasty (Trial 2)
|
Adverse Drug Reaction |
Placebo |
OLINVYK |
OLINVYK |
Morphineb |
|
(N = 79) |
(N = 79) |
(N = 79) |
(N = 76) |
|
|
Any TEAEC (%) |
78 |
94 |
95 |
98 |
|
Nausea |
46 |
62 |
75 |
74 |
|
Vomiting |
13 |
22 |
43 |
54 |
|
Hypoxia |
5 |
20 |
18 |
23 |
|
Constipation |
7 |
17 |
11 |
11 |
|
Pruritus |
5 |
17 |
11 |
18 |
|
Dizziness |
11 |
9 |
9 |
16 |
|
Sedation |
8 |
14 |
9 |
23 |
|
Back pain |
6 |
13 |
11 |
9 |
|
Somnolence |
1 |
0 |
5 |
7 |
b The morphine regimen included a loading dose of 4 mg, followed by access to a demand dose of 1 mg, with a 6-minute lockout period between doses, and 2-mg supplemental doses, beginning 1 hour after the initial dose, and hourly thereafter, as needed. c Treatment Emergent Adverse Event
Table 7. Adverse Drug Reactions Reported in ≥5% of OLINVYK -Treated Patients in Trial 3
|
Adverse Drug Reaction |
OLINVYK |
OLINVYK |
|
Patients with any TEAEa (%) |
62 |
69 |
|
Nausea |
29 |
38 |
|
Constipation |
10 |
13 |
|
Vomiting |
9 |
15 |
|
Headache |
4 |
5 |
|
Hypokalaemia |
4 |
7 |
|
Pruritus |
4 |
8 |
|
Pyrexia |
3 |
5 |
OLINVYK Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The overall occurrence of side effects was similar in men and women.
- Race: The overall occurrence of side effects was similar in White and Black or African American patients. The number of patients of other races was limited; therefore, differences in response for other races could not be determined.
- Age: The occurrence of side effects was similar across all tested age groups; however, the number of patients older than 65 years was limited
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize the occurrence of the adverse events by subgroup in pooled controlled trials (Trials 1 and 2).
Table 8. Adverse Events by Sex
|
|
Placebo |
OLINVYK |
Morphine |
|||
|
Women |
Men |
Women |
Men |
Women |
Men |
|
|
N (%) |
153 (94) |
9 (6) |
430 (91) |
40 (8) |
145 (92) |
13 (8) |
|
% Patients with at least 1 AE |
75 |
44 |
90 |
67 |
97 |
92 |
|
|
Percent Experiencing Selected AEs |
|||||
|
Preferred Term |
Women |
Men |
Women |
Men |
Women |
Men |
|
Nausea |
37 |
11 |
59 |
32 |
72 |
46 |
|
Vomiting |
10 |
0 |
32 |
15 |
53 |
38 |
|
Headache |
31 |
11 |
26 |
17 |
30 |
31 |
Table 9. Adverse Events by Race
|
|
Placebo |
OLINVYK |
Morphine N=158 |
|||
|
White |
AA |
White |
AA |
White |
AA |
|
|
N (%) |
110 (68) |
48 (30) |
310 (66) |
130 (27) |
104 (66) |
44 (28) |
|
% Patients with at least 1 AE |
75 |
69 |
89 |
86 |
98 |
93 |
|
|
Percent Experiencing Selected AEs |
|||||
|
Preferred Term |
White |
AA |
White |
AA |
White |
AA |
|
Nausea |
37 |
31 |
61 |
44 |
73 |
60 |
|
Vomiting |
10 |
10 |
34 |
20 |
49 |
58 |
|
Headache |
29 |
29 |
27 |
22 |
35 |
22 |
Table 10. Adverse Events by Age
|
|
≥18-<50 years |
≥50-<65 years |
≥65-<70 years |
≥70 years |
|||||||||
|
PI |
OL |
Mo |
PI |
OL |
Mo |
PI |
OL |
Mo |
PI |
OL |
Mo |
||
|
N (%) |
112 |
313 |
114 |
45 |
134 |
36 |
5 |
14 |
8 |
0 |
9 |
0 |
|
|
% Patients with at least 1 AE |
72 |
89 |
97 |
82 |
87 |
94 |
20 |
93 |
100 |
0 |
67 |
0 |
|
|
|
Percent Experiencing Selected AEs |
||||||||||||
|
≥18-<50 years |
≥50-<65 years |
≥65-<70 years |
≥70 years |
||||||||||
|
Preferred Term |
Pl |
OL |
Mo |
Pl |
OL |
Mo |
Pl |
OL |
Mo |
Pl |
OL |
Mo |
|
|
Nausea |
38 |
57 |
76 |
31 |
55 |
56 |
0 |
57 |
37 |
0 |
33 |
0 |
|
|
Vomiting |
10 |
32 |
53 |
11 |
29 |
44 |
0 |
36 |
62 |
0 |
22 |
0 |
|
|
Headache |
26 |
23 |
25 |
42 |
31 |
39 |
0 |
43 |
50 |
0 |
11 |
0 |
|
OL=OLINVYK,
Mo=morphine
Adapted from FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved OLINVYK based on evidence from three clinical trials (Trial 1/NCT02815709, Trial 2/NCT02820324 and Trial 3) of 1558 patients 18 to 89 years old who were in need for pain medication. The trials were conducted at 53 sites in the United States.
Patients from all three trials provided data for OLINVYK side effects and that population (safety population) is presented in the figures below. Separate trial populations are presented in Table 11 under MORE INFO.
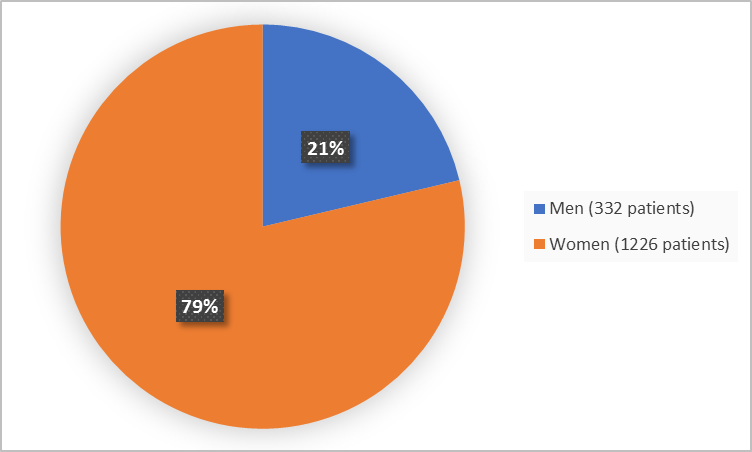
Figure 1 summarizes the percentage of patients by sex in the combined clinical trials.
Figure 1. Demographics by Sex (safety population)
FDA Review
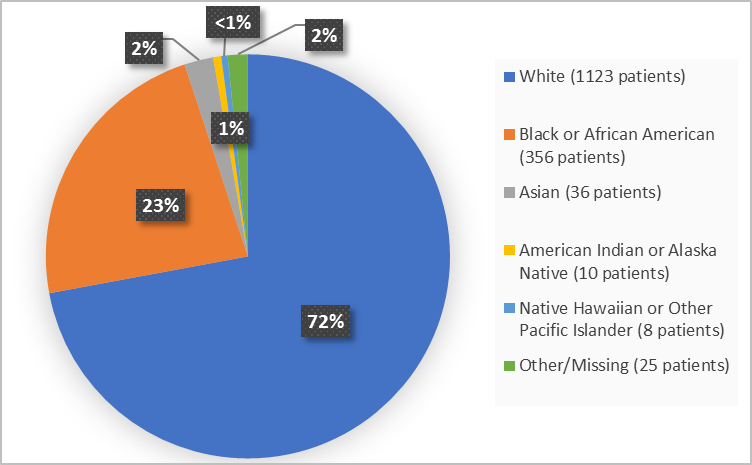
Figure 2 summarizes the percentage of patients by race in the in the combined clinical trials.
Figure 2. Demographics by Race (safety population)
FDA Review
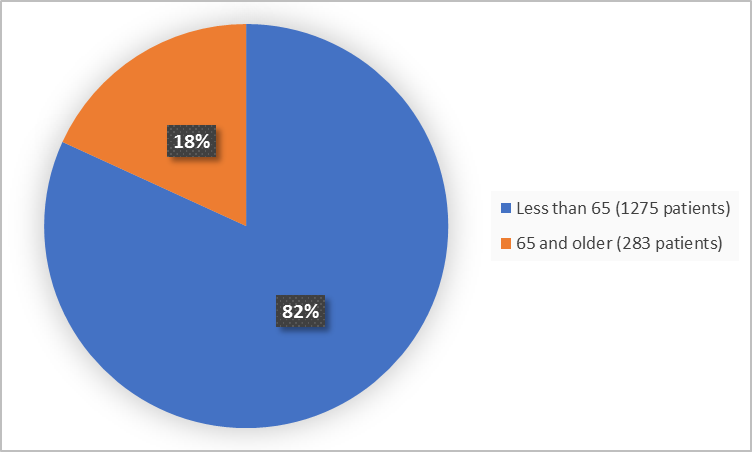
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Demographics by Age (safety population)
FDA Review
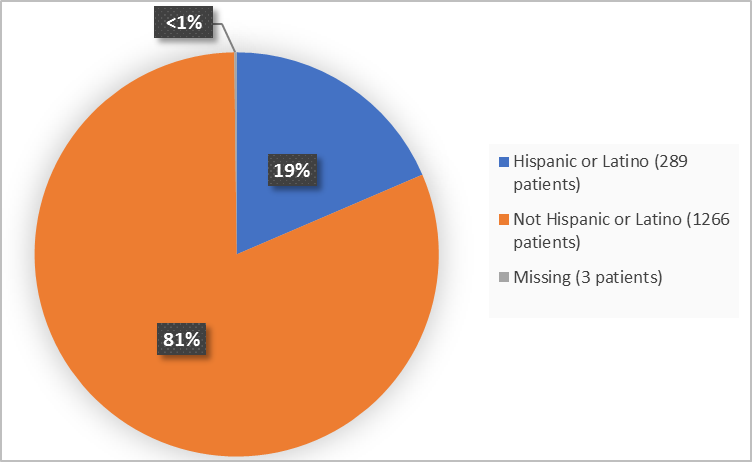
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trials.
Figure 4. Demographics by Ethnicity (safety population)
FDA Review
Who participated in the trials?
The table below summarizes demographics of all patients from the clinical trials.
Table 11. Demographic Characteristics for Trials 1, 2 and 3
|
Demographic Characteristics |
Trial 1 |
Trial 2 |
Trial 3 |
Total |
|
Sex |
|
|
|
|
|
Men |
59 (15.2) |
3 (0.7) |
270 (35.2) |
332 (21.3) |
|
Women |
330 (84.8) |
398 (99.3) |
498 (64.8) |
1226 (78.7) |
|
Race |
|
|||
|
White |
270 (69.4) |
257 (64.1) |
596 (77.6) |
1123 (72.1) |
|
Black or African American |
94 (24.2) |
125 (31.2) |
137 (17.8) |
356 (22.8) |
|
Asian |
14 (3.6) |
9 (2.2) |
13 (1.7) |
36 (2.3) |
|
American Indian or Alaska Native |
4 (1) |
1 (0.2) |
5(0.7) |
10 (0.6) |
|
Native Hawaiian or Other Pacific Islander |
4 (1) |
3 (0.7) |
1 (0.1) |
8 (0.5) |
|
Other |
3 (0.8) |
6 (1.5) |
14 (1.8) |
23 (1.5) |
|
Missing |
0 |
0 |
2 (0.3) |
2 (0.1) |
|
Age (years) |
|
|
|
|
|
Median (min, max) |
47 (19, 74) |
41 (20, 71) |
53 (18,89) |
48 (18,89) |
|
Age Group |
|
|||
|
18 to <50 years |
220 (56.6) |
319 (79.6) |
286 (37.2) |
825 (52.9) |
|
50 to <65 years |
143 (36.8) |
72 (17.9) |
235 (30.6) |
450 (28.9) |
|
65 to <70 years |
18 (4.6) |
9 (2.2) |
109 (14.2) |
136 (8.7) |
|
≥ 70 years |
8 (2) |
1 (0.2) |
138 (17.9) |
147 (9.4) |
|
Ethnicity |
|
|||
|
Hispanic or Latino |
96 (24.7) |
132 (32.9) |
61 (7.9) |
289 (18.5) |
|
Not Hispanic or Latino |
293 (75.3) |
269 (67.1) |
704 (91.7) |
1266 (81.3) |
|
Missing |
0 |
0 |
3 (0.4) |
3 (0.2) |
|
Region |
|
|||
|
United States |
389 (100) |
401 (100) |
768 (100) |
1558 (100) |
Clinical Trial Data
How were the trials designed?
Trials 1 enrolled patients who underwent bunion surgery. Patients with moderate to severe post-surgical pain were randomly assigned to receive OLINVYK, placebo or an approved drug to treat pain (morphine) for 48 hours through the vein. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed. All patients were allowed to use a rescue pain medication, if the pain was not well controlled using the trial medications.
Trial 2 enrolled patients who underwent surgical removal of abdominal wall fat (abdominoplasty) and had moderate to severe pain. Patients were randomly assigned to receive OLINVYK, placebo or an approved drug to treat pain (morphine) for 24 hours through the vein. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed. All patients were allowed to use a rescue pain medication, if the pain was not well controlled using the trial medications.
To assess the benefits of OLINVYK, patients used a numerical scale to score how severe the pain was after the surgery. The scores for the patients receiving OLINVYK were compared to the scores for the patients who received placebo and those who received morphine.
In the third trial, patients who had pain following various type of surgeries or due to a medical condition received at least one dose of OLINVYK. Data from this trial were used only to assess the side effects of OLINVYK.
How were the trials designed?
The efficacy and safety of OLINVYK were established in two randomized, double-blind, placebo-and active (morphine) -controlled trials. Trials 1 and 2 evaluated OLINVYK for the treatment of moderate to severe post-operative pain. Patients in Trial 1 had undergone a bunionectomy and received either placebo, morphine, OLINVYK 0.1 mg, OLINVYK 0.35 mg, or OLINVYK 0.5 mg for 48 hours. Patients in Trial 2 had undergone abdominoplasty and received either placebo, morphine, OLINVYK 0.1 mg, OLINVYK 0.35 mg, or OLINVYK 0.5 mg for 24 hours. If trial medication was inadequate, patients may have received rescue pain medication.
The analgesic effect was measured using the Summed Pain Intensity Differences over 48 hours (SPID-48) or 24 hours (SPID-24), respectively. The SPID is calculated by multiplying the Pain Intensity Difference (calculated by subtracting the pain intensity at a particular timepoint from the pain intensity at baseline) scores at each post-baseline timepoint by the duration (in hours) since the preceding timepoint, and then summing the values, over 48 or 24 hours.
Trial 3 was an open-label safety evaluation of OLINVYK at various doses in medical and surgical patients.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.