Drug Trials Snapshots: OCREVUS
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to OCREVUS Prescribing Information for complete information.
OCREVUS (ocrelizumab)
(ok’re vus)
Genentech
Approval date: March 28, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
OCREVUS is used for the treatment of patients with two types of multiple sclerosis (MS):
- relapsing forms of multiple sclerosis (RMS)
- primary progressive multiple sclerosis (PPMS).
In RMS patients have episodes of worsening function (relapses) followed by recovery periods. In PPMS patients have steadily worsening function from the onset of symptoms.
How is this drug used?
OCREVUS is given by a healthcare provider using a needle placed in a vein (known as intravenous infusion). The first two infusions are given 2 weeks apart followed by an infusion every 6 months. Before each infusion, patients should receive medicines to reduce the risk of infusion related allergic reactions.
What are the benefits of this drug?
Patients with RMS who received OCREVUS showed less frequent relapses in comparison to patients who received another MS drug, Rebif. Patients with PPMS who received OCREVUS experienced a longer period of time before worsening function compared to patients who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the Clinical Trials 1 and 2 conducted in in patients with RMS. The primary efficacy endpoint was the annualized relapse rate (ARR).
Table 3. Key Clinical and MRI Endpoints in RMS Patients from Trial 1 and Trial 2
| Endpoints | Trial 1 | Trial 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| OCREVUS 600 mg every 24 weeks N=410 |
Rebif 44 mcg three times a week N=411 |
OCREVUS 600 mg every 24 weeks N=417 |
Rebif 44 mcg three times a week N=418 |
|||||
| Clinical Endpoints | ||||||||
| Annualized Relapse Rate (Primary Endpoint1) | 0.156 | 0.292 | 0.155 | 0.290 | ||||
| Relative Reduction | 46% (p<> | 47% (p<> | ||||||
| Proportion Relapse-free | 83% | 71% | 82% | 72% | ||||
| Proportion of Patients with 12-week Confirmed Disability Progression1 | 9.8% OCREVUS vs 15.2% REBIF | |||||||
| Risk Reduction (Pooled Analysis2) | 40%; p=0.0006 | |||||||
| MRI Endpoints | ||||||||
| Mean number of T1 Gd-enhancing lesions per MRI scan | 0.016 | 0.286 | 0.021 | 0.416 | ||||
| Relative Reduction | 94% (p<> | 95% (p<> | ||||||
| Mean number of new and/or enlarging T2 hyperintense lesions per MRI | 0.323 | 1.413 | 0.325 | 1.904 | ||||
| Relative Reduction | 77% (p<> | 83% (p<> | ||||||
1Defined as an increase of 1 point or more from the baseline Expanded Disability Status Scale (EDSS) score for patients with baseline score of 5.5 or less or 0.5 or more when the baseline score is greater than 5.5, Kaplan-Meier estimates at Week 96
2Data prospectively pooled from Trial 1and Trial 2
OCREVUS Prescribing Information
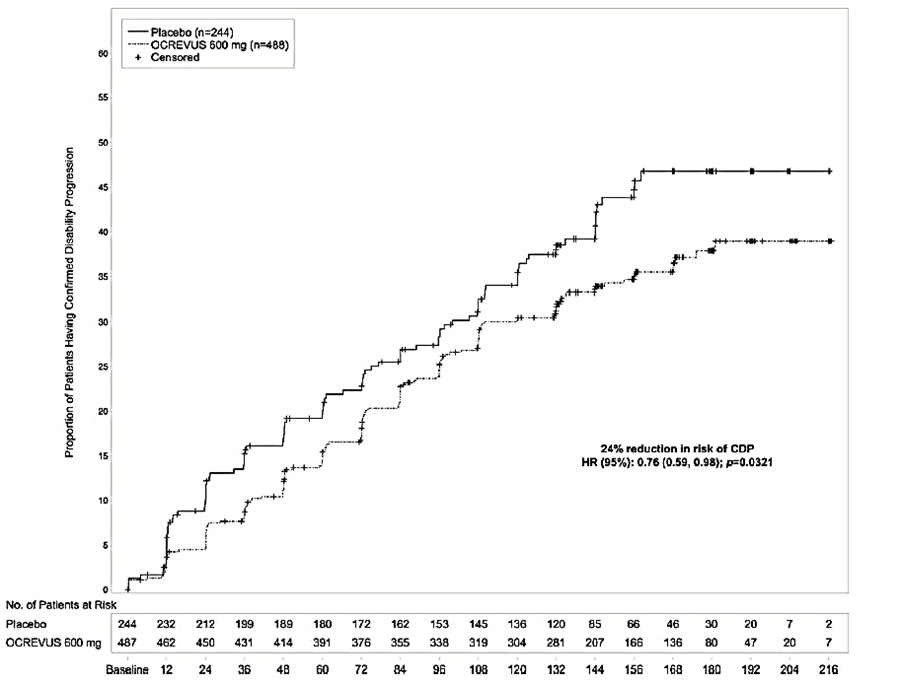
Efficacy results of Trial 3 in patients with PPMS are presented in Table 4 and Figure 7. The primary efficacy endpoint was the time to onset of confirmed disability progression (CDP) attributable to MS confirmed to be present at the neurological assessment at least 12 weeks later.
Table 4. Key Clinical and MRI Endpoints in PPMS patients for Trial 3
| Endpoints | Trial 3 | |
|---|---|---|
| OCREVUS 600 mg (two 300 mg infusions two weeks apart every 24 weeks) N=488 |
Placebo N=244 |
|
| Clinical Outcomes | ||
| Proportion of patients with 12-week Confirmed Disability Progression1 (Primary Endpoint) | 32.9 % | 39.3% |
| Risk reduction | 24%; p=0.0321 | |
| MRI Endpoints | ||
| Mean change in volume of T2 lesions, from baseline to Week 120 (cm3) | -0.393 | 0.788 |
| (p<> | ||
1Defined as an increase of 1 point or more from the baseline EDSS score for patients with baseline score or 5.5 or less, or an increase of 0.5 or more when the baseline score is more than 5.5
OCREVUS Prescribing Information
Figure 7. Kaplan-Meier Plot of Time to Onset of Confirmed Disability Progression Sustained for at Least 12 Weeks with the Initial Event of Neurological Worsening Occurring During the Double-blind Treatment Period in Trial 3*
*All patients in this analysis had a minimum of 120 weeks of follow-up. The primary analysis is based on all disability progression events accrued including21 without confirmatory EDSS at 12 weeks.
OCREVUS Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: OCREVUS worked similarly in men and women with RMS, but better in men with PPMS.
- Race: Most of the patients were White. The number of patients of other races was limited; therefore differences in response to OCREVUS among races could not be determined.
- Age: OCREVUS worked similarly in age groups studied. The number of patients above 65 years of age was limited, however, so that differences in response between patients above and below 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table below summarizes primary efficacy results by age and sex subgroups in Trials 1 and 2 separately.
Table 5. Subgroup Analysis of ARR by Age and Sex (Trials 1 and 2)
| Trial 1 | Trial 2 | ||||
|---|---|---|---|---|---|
| Rebif N=410 |
OCREVUS N=410 |
Rebif N=418 |
OCREVUS N=417 |
||
| Age Group (years) | |||||
| <> | |||||
| N | 242 | 244 | 241 | 252 | |
| ARR | 0.358 | 0.152 | 0.362 | 0.146 | |
| Rate Ratio | 0.423 | 0.403 | |||
| > 40 | |||||
| N | 168 | 166 | 177 | 165 | |
| RR | 0.245 | 0.169 | 0.214 | 0.173 | |
| Rate Ratio | 0.692 | 0.808 | |||
| Sex | |||||
| Women | |||||
| N | 271 | 270 | 280 | 271 | |
| ARR | 0.306 | 0.152 | 0.301 | 0.165 | |
| Rate Ratio | 0.498 | 0.547 | |||
| Men | |||||
| N | 139 | 140 | 138 | 146 | |
| ARR | 0.267 | 0.162 | 0.231 | 0.120 | |
| Rate Ratio | 0.607 | 0.520 | |||
ARR=Annualized Relapse Rate
FDA Statistical review
Table below summarizes primary efficacy results by age and sex subgroups in Trial 3.
Table 6. Subgroup Analysis of 12-week CDP by Age and Sex (Trial 3)
| Placebo N=244 |
OCREVUS N=487 |
||||
|---|---|---|---|---|---|
| Age Group (years) | |||||
| ≤45 | |||||
| N | 118 | 230 | |||
| Number (%) of CDP | 49 (41.5%) | 71 (30.9%) | |||
| Hazard Ratio | 0.641 | ||||
| >45 | |||||
| N | 126 | 257 | |||
| Number (%) of CDP | 47 (37.3%) | 89 (34.6%) | |||
| Hazard Ratio | 0.884 | ||||
| Sex | |||||
| Women | |||||
| N | 124 | 236 | |||
| Number (%) of CDP | 44 (35.5%) | 85 (36.0%) | |||
| Hazard Ratio | 0.944 | ||||
| Men | |||||
| N | 120 | 251 | |||
| Number (%) of CDP | 52 (43.3%) | 75 (29.9%) | |||
| Hazard Ratio | 0.614 | ||||
CDP=confirmed disability progression
FDA Statistical review
What are the possible side effects?
OCREVUS may frequently cause infusion reactions, some of which can be severe causing rash, itching, trouble breathing, or fall in blood pressure. In trials of OCREVUS, about 35-40% of patients experienced an infusion reaction after being given a corticosteroid and an antihistamine with the infusion. This reduced the severity of the infusion reactions, but they still occurred.
Other serious side effects of OCREVUS are increased risk of infections and increased risk of cancers.
Apart from infusion reactions, the most common side effects of OCREVUS are upper and lower respiratory infections and skin infections.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in at least 5% of OCREVUS treated patients in pooled trials of patients with RMS.
Table 7. Adverse Reactions in Adult Patients with RMS with an Incidence of at least 5% for OCREVUS and Higher than Rebif
| Adverse Reactions | Trials 1 and 2 | |
|---|---|---|
| OCREVUS 600 mg IV Every 24 Weeks1 (n=825) % |
Rebif 44 mcg SQ 3 Times per Week (n=826) % |
|
| Upper respiratory tract infections | 40 | 33 |
| Infusion reactions | 34 | 10 |
| Depression | 8 | 7 |
| Lower respiratory tract infections | 8 | 5 |
| Back pain | 6 | 5 |
| Herpes virus- associated infections | 6 | 4 |
| Pain in extremity | 5 | 4 |
1 The first dose was given as two separate 300 mg infusions at Weeks 0 and 2.
Table 8. Adverse Reactions in Adult Patients with PPMS with an Incidence of at least 5% for OCREVUS and Higher than Placebo
| Adverse Reactions | Trial 3 | |
|---|---|---|
| OCREVUS 600 mg IV Every 24 Weeks1 (n=486) % |
Placebo (n=239) % |
|
| Upper respiratory tract infections | 49 | 43 |
| Infusion reactions | 40 | 26 |
| Skin infections | 14 | 11 |
| Lower respiratory tract infections | 10 | 9 |
| Cough | 7 | 3 |
| Diarrhea | 6 | 5 |
| Edema peripheral | 6 | 5 |
| Herpes virus associated infections | 5 | 4 |
1 One dose of OCREVUS (600 mg administered as two 300 mg infusions two weeks apart)
Were there any differences in side effects among sex, race and age?
- Sex: The risk of side effects was similar in in men and women.
- Race: Majority of the patients were White. The number of patients in other races was limited; therefore differences in side effects among races could not be determined.
- Age: The risk of side effects was similar in age groups studied. The number of patients above 65 years of age was limited; therefore, differences in response between patients above and below 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Tables below summarize selected safety results by sex and age subgroups.
Table 9. Select Adverse Events by Sex (safety population Trials 1 and 2)
| Outcome | Rebif | OCREVUS | ||
|---|---|---|---|---|
| Men 468PYs n=277 |
Women 931PYs n=549 |
Men 501PYs n=248 |
Women 947PYs n=541 |
|
| AE | 255.5/100PY (n=1196) |
316.4/100PYs (n=2945) |
258.1/100PYs (n=1294) |
306.4/100PYs (n=2900) |
| SAE | 7.1/100PYs (n=33) |
5.9/100PYs (n=55) |
6.4/100PYs (n=32) |
4.9/100PY (n=46) |
| Infection | 57.3/100PY (n=268) |
75.0/100PYs (n=698) |
63.8/100PYs (n=320) |
96.9/100PYs (n=917) |
| Malignancy | (n=0) | 0.2/100PYs (n=2) |
0.4/100PYs (n=2) |
0.2/100PY (n=2) |
PYc=patient years AE-adverse event
SAE=serious adverse event
Adapted from FDA clinical review
Table 10. Select Adverse Events by Age Group (safety population Trials 1 and 2)
| Outcome | Rebif | OCREVUS | ||
|---|---|---|---|---|
| Age<=45> 1105PYs n=652 |
Age>45 294PYs n=174 |
Age<=45> 1136PYs n=648 |
Age>45 312PY n=177 |
|
| AE | 293.7/100PYs (n=3245) |
304.8/100PYs (n=896) |
298.9/100PYs (n=3395) |
256.1/100PYs (n=799) |
| SAE | 5.8/100PYs (n=64) |
8.2/100PYs (n=24) |
4.6/100PYs (n=52) |
8.3/100PYs (n=26) |
| Infection | 70.3/100PYs (n=777) |
64.3/100PYs (n=189) |
89.3/100PYs (n=1014) |
71.5/100PYs (n=223) |
| Malignancy | (n=0) | 0.7/100PYs | 0.2/100PYs | 0.6/100PYs |
PYc=patient years AE-adverse event
SAE=serious adverse event
Adapted from FDA clinical review
Table 11. Select Adverse Events by Sex (safety population Trial 3)
| Outcome | Placebo | OCREVUS | ||
|---|---|---|---|---|
| Men 325PYs n=120 |
Women 335PYs n=119 |
Men 706PYs n=246 |
Women 710PYs n=240 |
|
| AE | 256.0/100PY (n=832) |
277.8/100PYs (n=930) |
260.7/100PYs (n=1842) |
260.3/100PYs (n=1848) |
| SAE | 13.2/100PYs (n=43) |
10.2/100PYs (n=34) |
10.5/100PYs (n=74) |
10.0/100PY (n=71) |
| Infection | 68.3/100PY (n=222) |
83.6/100PYs (n=280) |
69.5/100PYs (n=491) |
83.5/100PYs (n=593) |
| Malignancy | (n=0) | 0.6/100PYs (n=2) | 0.9/100PYs (n=6) | 1.0/100PY (n=7) |
PYc=patient years
AE-adverse event
SAE=serious adverse event
Adapted from FDA clinical review
Table 12. Select Adverse Events by Age Groups (safety population Trial 3)
| Outcome | Placebo | OCREVUS | ||
|---|---|---|---|---|
| Age<=45> 304PYs n=113 |
Age>45 356PYs n=126 |
Age<=45> 677PYs n=230 |
Age>45 739PY n=256 |
|
| AE | 261/100PYs (n=793) |
272/100PYs (n=969) |
237/100PYs (n=1602) |
283/100PYs (n=2088) |
| SAE | 7.9/100PYs (n=24) |
14.9/100PYs (n=53) |
8.3/100PYs (n=56) |
12.0/100PYs (n=89) |
| Infection | 77.3/100PYs (n=235) |
75.0/100PYs (n=267) |
66.6/100PYs (n=451) |
85.7/100PYs (n=633) |
| Malignancy | (n=0) | 0.6/100PYs (n=2) |
0.1/100PYs (n=1) |
1.6/100PYs (n=12) |
PYc=patient years AE-adverse event
SAE=serious adverse event
Adapted from FDA clinical review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved OCREVUS based on evidence from three clinical trials of patients with MS.
Two trials (Trials 1 and 2) enrolled total of 1656 patients with RMS in US, Canada, European countries, Latin America, Africa and Australia. They compared OCREVUS to Rebif.
One trial (Trial 3) enrolled total of 732 patients with PPMS in USA, Canada and European countries. This trial compared OCREVUS to placebo.
These two groups of patients will be presented separately.
The figure below summarizes how many men and women were enrolled in Clinical Trials 1 and 2.
Figure 1. Baseline Demographics by Sex (Trials 1 and 2 combined)
Adapted from FDA Clinical review
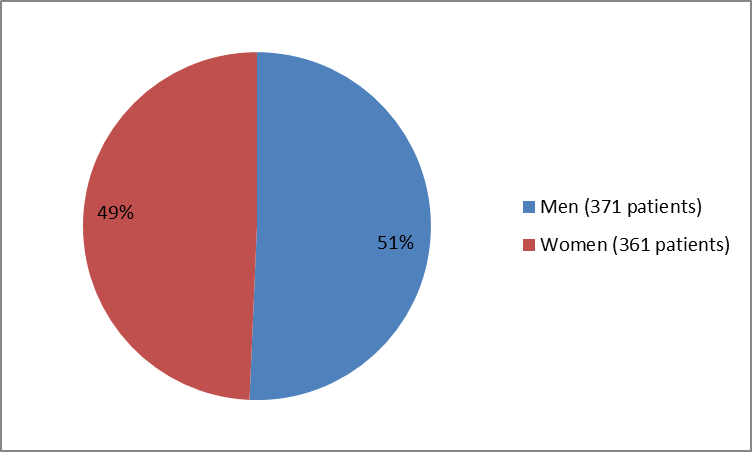
Figure 2. Baseline Demographics by Sex (Trial 3)
Adapted from FDA Clinical review
Figure 3 and Table 1 below summarize the percentage of patients by race in Clinical Trials 1 and 2 combined.
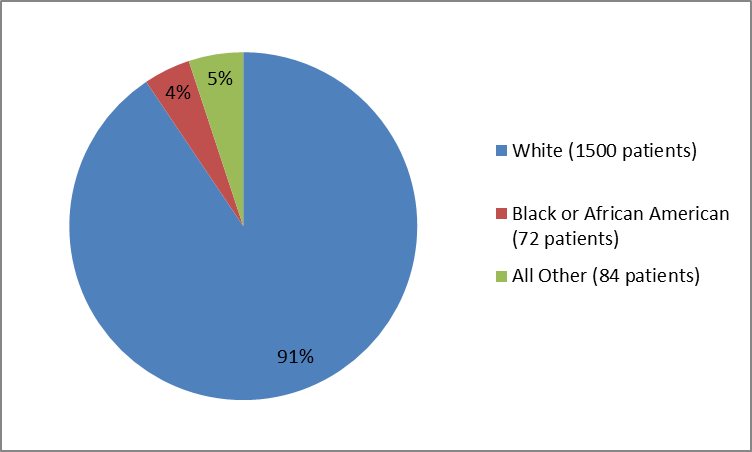
Figure 3. Baseline Demographics by Race (Trials 1 and 2 combined)
Adapted from FDA Clinical review
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 1500 | 91 |
| Black or African American | 72 | 4 |
| Other | 84 | 5 |
Adapted from FDA Clinical review
Figure 4 and Table 2 below summarize the percentage of patients by race in Clinical Trial 3.
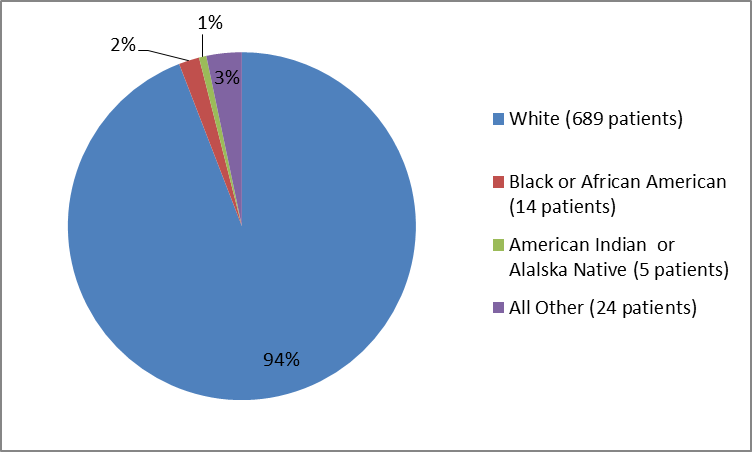
Figure 4. Baseline Demographics by Race (Trial 3)
Adapted from FDA Clinical review
Table 2. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 689 | 94 |
| Black or African American | 14 | 2 |
| American Indian or Alaska Native | 5 | 1 |
| Unknown | 2 | less than 1% |
| Other | 22 | 3 |
Adapted from FDA Clinical review
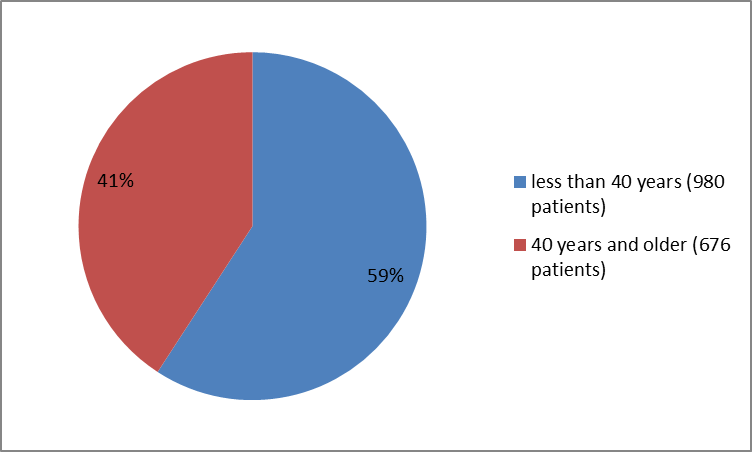
Figure 5 summarizes the percentage of patients by age in Clinical Trials 1 and 2 combined.
Figure 5. Baseline Demographics by Age (Trials 1 and 2 combined)
Adapted from FDA Clinical review
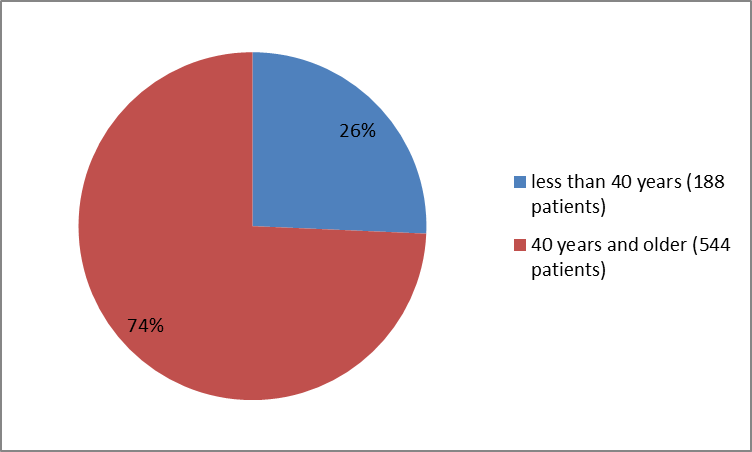
Figure 6 summarizes the percentage of patients by age in Clinical Trial 3.
Figure 6. Baseline Demographics by Age (Trial 3)
Adapted from FDA Clinical review
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials.
Table 13. Baseline Demographics of Patients in the Clinical Trials 1 and 2 (ITT population)
| Demographic parameter | OCREVUS N=827 |
Rebif N=829 |
Total N=1656 |
|---|---|---|---|
| Age | |||
| Median (Min, Max) | 38 (18, 56) | 38 (18,55) | 38 (18, 56) |
| Age Group | |||
| <40> | 496(60) | 484 (58) | 980 (59) |
| ≥40 years | 331(40) | 345 (42) | 676(41) |
| Sex, n (%) | |||
| Men | 277 (34%) | 286 (35%) | 563 (34) |
| Women | 552 (67%) | 541 (65%) | 1093 (66) |
| Race, n (%) | |||
| White | 743 (89%) | 757 (91%) | 1500 (91) |
| Black or African American | 40 (5) | 32 (4) | 72 (4) |
| All Other | 44 (5%) | 40 (5%) | 84 (5) |
| Geographic Region, n (%) | |||
| USA | 217 (26%) | 219 (26%) | 436 (26) |
| All Other | 610 (74%) | 610 (74%) | 1220 (74) |
Adapted from FDA Clinical review
Table 14. Baseline Demographics of Patients in the Clinical Trial 3 (ITT population)
| Demographic parameter | OCREVUS N=488 |
Placebo N=244 |
Total N=732 |
|---|---|---|---|
| Age | |||
| Median (Min, Max) | 46 (20,56) | 46 (18, 55) | 46 (18, 56) |
| Age Group | |||
| <40> | 120 (25) | 68 (28) | 188 (26) |
| ≥40 years | 368 (75) | 176 (72) | 544 (74) |
| Sex, n (%) | |||
| Men | 251 (51) | 120 (49) | 371 (51) |
| Women | 237 (49) | 124 (51) | 361 (49) |
| Race, n (%) | |||
| White | 454 (93) | 235 (96) | 689 (94) |
| Black or African American | 9 (2) | 5 (2) | 14 (2) |
| American Indian or Alaska Native | 5 (1) | 0 | 5 (1) |
| Unknown | 2 (<> | 0 | 2 (<> |
| Other | 18 (4) | 4 (2) | 22 (3) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 385 (79) | 206 (84) | 591(81) |
| Hispanic or Latino | 32 (7) | 14 (6) | 46 (6) |
| Not reported | 51 (11) | 16 (7) | 67 (9) |
| Unknown | 18 (4) | 8 (3) | 26 (4) |
| Missing | 2 (<> | 0 | 2 (<> |
| Geographic Region, n (%) | |||
| USA | 67 (14) | 34 (14) | 101 (14) |
| Other | 421 (86%) | 210 (86) | 631 (86) |
Adapted from FDA Clinical review
How were the trials designed?
The benefits and side effects of OCREVUS for RMS were established in two clinical trials of patients with MS who had experienced at least one relapse within the prior year, or two relapses within the prior two years.
Patients received treatment with either OCREVUS or another MS drug, Rebif, for 96 weeks. Neither the patients nor the health care providers knew which treatment was being given until the trial was completed.
The benefit of OCREVUS was evaluated by comparing the yearly relapse rate between the groups.
The benefits and side effects of OCREVUS for PPMS were evaluated in one clinical trial of patients with a certain pre-determined degree of disability measured by Expanded Disability Status Scale (EDSS). Patients received treatment with either OCREVUS or placebo for at least 120 weeks. Neither the patients nor the health care providers knew which treatment was being given until the trial was completed.
The benefit of OCREVUS was evaluated by comparing the time to disability progression in each group through neurological examination. Disability progression occurred when the baseline degree of disability (EDSS score) increased by 1 point or more from the baseline EDSS if the baseline EDSS was 5.5 points or less or by 0.5 points or more if the baseline EDSS was more than 5.5 points. To count as progression the observed disability had to last for 12 weeks.
How were the trials designed?
The safety and efficacy of OCREVUS for RMS was established in two randomized, double-blind, double-dummy, active comparator-controlled clinical trials of 96 weeks duration. Trials included patients who had experienced at least one relapse within the prior year, or two relapses within the prior two years, and had an Expanded Disability Status Scale (EDSS) score from 0 to 5.5. The primary outcome was the annualized relapse rate (ARR).
The safety and efficacy of OCREVUS for PPMS was established in one randomized, double-blind, placebo-controlled clinical trial of 120 weeks duration. Trial included patients with a baseline EDSS of 3 to 6.5 and a score of 2 or greater for the EDSS pyramidal functional system due to lower extremity findings.
The primary outcome was the time to onset of disability progression attributable to MS confirmed to be present at the next neurological assessment at least 12 weeks later. Disability progression occurred when the EDSS score increased by 1 point or more from the baseline EDSS if the baseline EDSS was 5.5 points or less or by 0.5 points or more if the baseline EDSS was more than 5.5 points.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION