Drug Trials Snapshots: MAYZENT
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights individuals who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the MAYZENT Package Insert for complete information.
MAYZENT (siponimod)
mā’zĕnt
Novartis Pharmaceuticals Corporation
Approval date: March 26, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MAYZENT is a drug used for the treatment of adults with relapsing forms of multiple sclerosis (RMS) including:
- clinically isolated syndrome,
- relapsing-remitting disease, and
- active secondary progressive disease
In RMS, patients have episodes of worsening function (relapses) followed by recovery periods. Patients can also experience an increase in the underlying disability, particularly as the disease progresses.
How is this drug used?
MAYZENT is taken by mouth once daily. MAYZENT is initially started at a low dose. The dose is gradually increased over 5 to 6 days.
What are the benefits of this drug?
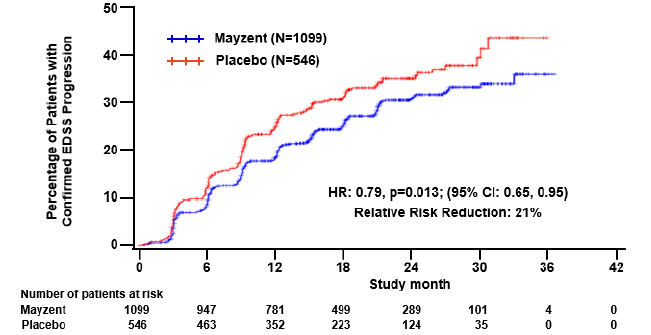
MAYZENT reduced the risk of disability progression. Twenty-six percent of patients that received MAYZENT had confirmed progression of disability that was sustained over a 3-month period compared to 32% of patients that received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the evaluated patients in the clinical efficacy trial. The primary endpoint was time to confirmed disability progression (CDP) based on worsening of EDSS ratings that were confirmed at two examinations, 3-months apart.
Figure 4. Time to Confirmed Disability Progression Based on EDSS (Trial 1)
MAYZENT Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: MAYZENT worked similarly in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in how well MAYZENT worked among races could not be determined.
- Age: MAYZENT worked similarly in patients younger and older than 45 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by sex, race and age in Trial 1.
Table 2. Trial 1: Confirmed Disability Progression by Demographic Subgroups
| Subgroup Category | Number of patients randomized | Number (%) of patients with confirmed disability progression | Hazard ratio MAYZENT vs placebo (95% confidence interval) |
|||
|---|---|---|---|---|---|---|
| MAYZENT | placebo | MAYZENT | placebo | |||
| Sex | Men | 435 | 223 | 129 (30) | 75 (34) | 0.81 (0.60, 1.07) |
| Women | 664 | 323 | 159 (24) | 98 (30) | 0.77 (0.60, 1.00) | |
| Race | White | 1046 | 513 | 275 (26) | 168 (33) | 0.75 (0.62, 0.92) |
| Other | 53 | 33 | 13 (25) | 5 (15) | 2.06 (0.72, 5.86) | |
| Age Group | <45 years | 393 | 199 | 111 (28) | 74 (37) | 0.74 (0.55, 0.99) |
| >45 years | 706 | 347 | 177 (25) | 99 (29) | 0.83 (0.65, 1.06) | |
FDA Review
What are the possible side effects?
MAYZENT may cause serious side effects including infection, build-up of fluid in the back of the eye (macular edema), decreased heart rate, decrease in lung function, liver injury, increased blood pressure, and risk to a fetus.
The most common side effects of MAYZENT are headache, high blood pressure, and increased liver function tests.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in patients with SPMS in the clinical trial.
Table 3. Adverse Reactions Reported in Trial 1 (Occurring in at Least 5% of MAYZENT-Treated Patients and at a Rate at Least 1% Higher Rate Than in Patients Receiving Placebo)
| Primary System Organ Class Adverse Reaction |
MAYZENT 2 mg N = 1099 % |
Placebo N = 546 % |
|---|---|---|
| Headachea | 15 | 14 |
| Hypertensionb | 13 | 9 |
| Transaminase increasedc | 11 | 3 |
| Falls | 11 | 10 |
| Edema peripherald | 8 | 4 |
| Nausea | 7 | 4 |
| Dizziness | 7 | 5 |
| Diarrhea | 6 | 4 |
| Bradycardiae | 6 | 3 |
| Pain in extremityf | 6 | 4 |
Terms were combined as follows:
a headache, tension headache, sinus headache, cervicogenic headache, drug withdrawal headache, and procedural headache
b hypertension, blood pressure increased, blood pressure systolic increased, essential hypertension, blood pressure diastolic increased
c alanine aminotransferase increased, gamma-glutamyltransferase increased, hepatic enzyme increased, aspartate aminotransferase increased, blood alkaline phosphatase increased, liver function test increased, hepatic function abnormal, liver function test abnormal, transaminases increased
d edema peripheral, joint swelling, fluid retention, swelling face
e bradycardia, sinus bradycardia, heart rate decreased
f pain in extremity and limb discomfort
MAYZENT Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 45 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the most common adverse reaction, headache, by subgroup.
Table 4. Subgroup Analysis of Headache
| Demographic Characteristics | MAYZENT n/N (%) |
Placebo n/N (%) |
|---|---|---|
| Sex, n (%) | ||
| Men | 52/435 (12.0) | 29/223 (13.0) |
| Women | 122/664 (18.4) | 53/323 (16.4) |
| Race, n (%) | ||
| White | 164/1046 (15.7) | 78/513 (15.2) |
| Black or African American | 2/7 (28.6) | 0/3 (0) |
| Asian | 3/30 (10.0) | 3/18 (16.7) |
| Other | 2/11 (18.2) | 1/7 (14.3) |
| Age Group, n (%) | ||
| < 45 years | 58/393 (14.8) | 28/199 (14.1) |
| > 45 years | 116/706 (16.4) | 54/347 (15.6) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved MAYZENT based on evidence primarily from one clinical trial (Trial 1/NCT01665144) of 1651 patients with secondary progressive multiple sclerosis (SPMS). The trial was conducted at 294 centers in Asia, Australia, Canada, Europe, South America, and the United States.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Demographics by Sex
FDA Review
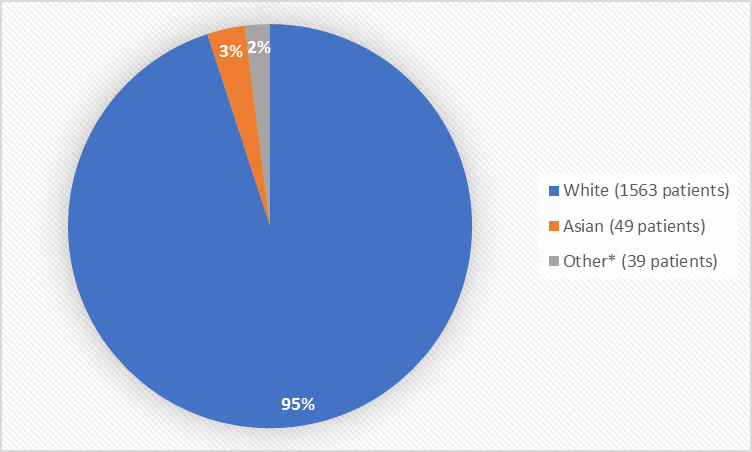
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trial.
Figure 2. Demographics by Race
*Includes Black or African American
FDA Review
Table 1. Demographics of Trials by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 1563 | 95% |
| Black or African American | 10 | Less than 1% |
| Asian | 49 | 3% |
| Other | 29 | 2% |
FDA Review
Figure 3 summarizes the percentage of patients by age group in the clinical trial.
Figure 3. Demographics by Age
FDA Review
Who participated in the trials?
The table below summarizes the demographics of the randomized patients in the clinical trials.
Table 5. Demographics of Patients in the Clinical Trials
| Demographic Parameters | MAYZENT (n=1105) | Placebo (n=546) | Total = 1651 |
|---|---|---|---|
| Sex | |||
| Men | 436 (39%) | 223 (41%) | 659 (40%) |
| Women | 669 (61%) | 323 (59%) | 992 (60%) |
| Race | |||
| White | 1050 (95.0%) | 513 (94.0%) | 1563 (94.7%) |
| Black or African American | 7 (0.6%) | 3 (0.5%) | 10 (0.6%) |
| Asian | 31 (2.8%) | 18 (3.3%) | 49 (3.0%) |
| Other/Unknown | 17 (1.5%) | 12 (2.2%) | 29 (1.7%) |
| Age group | |||
| ≤45 years | 394 (35.7%) | 199 (36.5%) | 593 (35.9%) |
| >45 years | 711 (64.3%) | 347 (63.6%) | 1058 (64.1%) |
| Age (years) | |||
| Mean (SD) | 48.0 (7.8) | 48.1 (7.9) | 48.0 (7.8) |
| Median | 49 | 49 | 49 |
| Min, max | 22, 61 | 21, 61 | 21, 61 |
| Ethnicity | |||
| Hispanic or Latino | 74 (6.7%) | 32 (5.9%) | 106 (6.4%) |
| Not Hispanic or Latino | 829 (75.0%) | 410 (75.1%) | 1239 (75.0%) |
| Other/Unknown | 202 (18.3%) | 104 (19.1%) | 306 (18.5%) |
| Region | |||
| United States | 102 (9.2%) | 52 (9.5%) | 154 (9.3%) |
| Rest of the World | 1003 (90.8%) | 494 (90.5%) | 1497 (90.7%) |
| Canada | 32 (2.9%) | 15 (2.7%) | 47 (2.8%) |
| South America | 20 (1.8%) | 10 (1.8%) | 30 (1.8%) |
| Europe | 810 (73.3%) | 401 (73.4%) | 1211 (73.3%) |
| Asia | 117 (10.6%) | 56 (10.3%) | 173 (10.5%) |
| Australia | 24 (2.2%) | 12 (2.2%) | 36 (2.2%) |
FDA Review
How were the trials designed?
The benefits and side effects of MAYZENT were evaluated in one clinical trial of patients with SPMS who had experienced disability progression in the two years prior to enrollment and no relapses in the three months prior to enrollment. The degree of disability was established by neurologic examination using the Expanded Disability Status Scale (EDSS). The EDSS is used to score disability on a scale from 0 (normal) to 10 (death due to MS). Patients received MAYZENT or placebo tablets by mouth once daily for up to 37 months. Neither the patients nor the health care providers knew which treatment was being given until the trial was completed.
The benefit of MAYZENT was evaluated by comparing the time to disability progression in each group using EDSS scores. To count as progression, the observed disability progression had to last for 3 months.
How were the trials designed?
The safety and efficacy of MAYZENT were established primarily in one randomized, double-blind, placebo-controlled trial. The trial enrolled patients with SPMS who met the following criteria: evidence in disability progression in the prior 2 years, no evidence of relapse in the 3 months prior to trial enrollment, and an EDSS score of 3.0 to 6.5 at trial entry. The EDSS is used to assess disability on a scale from 0 (normal) to 10 (death due to MS). Patients were randomized to receive once daily MAYZENT 2 mg orally or placebo after initial drug titration for Days 1 to 6. Patients received MAYZENT or placebo for up to 37 weeks.
The primary endpoint was the time to confirmed disability progression (CDP) attributable to MS, defined as at least 1-point increase from baseline in EDSS (0.5-point increase for patients with baseline EDSS of 5.5 or higher) sustained for 3 months.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.