Drug Trials Snapshots: LUTATHERA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the LUTHATERA Package Insert for complete information.
LUTATHERA (lutetium 177 dotate)
{Lou-Ta-ther-a}
Advanced Accelerator Applications, S.A.
Approval date: January 26, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
LUTATHERA is a drug for the treatment of adults who have a specific type of tumor called a gastroenteropancreatic neuroendocrine tumor (GEP-NET). NETs are rare tumors that develop in certain hormone-producing cells of the body.
LUTATHERA is intended for the treatment of throat, esophagus, stomach, and intestine neuroendocrine tumors that have somatostatin receptors.
How is this drug used?
LUTATHERA is given by a healthcare provider directly into the bloodstream. This is known as an intravenous or IV infusion. It takes about 30 minutes to receive a LUTATHERA infusion.
LUTATHERA is given once every 8 weeks for a total of 4 doses.
What are the benefits of this drug?
LUTATHERA increased the length of time tumors did not grow after treatment (progression free survival).
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the clinical trial NETTER-1 in patients with somatostatin-receptor positive midgut GEP-NETs. The primary endpoint was progression free survival, according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1., from the date of randomization to tumor progression.
Table 2. Efficacy Results in NETTER-1
| LUTATHERA and Long-Acting Octreotide (30 mg) N=116 |
Long-Acting Octreotide (60 mg) N=113 |
|
|---|---|---|
| PFS by IRC | ||
| Events (%) | 27 (23%) | 78 (69%) |
| Progressive Disease, n (%) | 15 (13%) | 61 (54%) |
| Death, n (%) | 12 (10%) | 17 (15%) |
| Median in months (95% CI) | NRc (NE, NE) | 8.5 (5.8, 9.1) |
| Hazard ratioa (95% CI) | 0.21 (0.13, 0.32) | |
| P-Valueb | <> | |
| OS (Updated) | ||
| Deaths (%) | 27 (23%) | 43 (38%) |
| Median in months (95% CI) | NR (31.0, NE) | 27.4 (22.2, NE) |
| Hazard ratioa,d (95% CI) | 0.52 (0.32, 0.84) | |
| ORR by IRC | ||
| ORR, % (95% CI) | 13% (7%,19%) | 4 (0.1%, 7%) |
| Complete response rate, n (%) | 1 (1%) | 0 |
| Partial response rate, n (%) | 14 (12%) | 4 (4%) |
| P-Valuee | 0.0148 | |
| Duration of response, median in months (95% CI) | NR (2.8, NE) | 1.9 (1.9, NE) |
a: Hazard ratio based on the unstratified Cox model
b: Unstratified log rank test
c: Median follow-up 10.5 months at time of primary analysis of PFS (0 to 29 months)
d: Interim analysis of OS not statistically significant based on pre-specified significance criteria
e: Fisher’s Exact test
NR: Not reached; NE: Not evaluable
LUTATHERA Prescribing Information
Efficacy in the ERASMUS study was assessed, in a subset of 360 patients with GEP-NETs, whose tumors were assessed based on standardized imaging criteria called RECIST.The investigator-assessed overall response rate (ORR) was 16% (95% CI 13, 20). Three complete responses were observed (< 1%). Median duration of response (DoR) in the 58 responding patients was 35 months (95% CI: 17, 38).
LUTATHERA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: LUTATHERA worked similarly in men and women.
- Race: Most of the patients were White. Differences in how well the drug worked among races could not be determined because of the small number of patients in other races.
- Age: LUTATHERA worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below presents efficacy results by subgroup in NETTER-1 trial.
Table 3. NETTER-1 Trial: Subgroup Analyses
| Sample Size (Censored/Events) | Un-stratified HR (95% CI) |
||
|---|---|---|---|
| LUTATHERA + Octreotide LAR (30 mg) N=116 |
Octreotide LAR (60 mg) N=113 |
||
| Overall | 89/27 | 35/78 | 0.21 (0.13, 0.32) |
| Sex | |||
| Men | 46/17 | 16/37 | 0.24 (0.13, 0.43) |
| Women | 43/10 | 19/41 | 0.16 (0.08, 0.33) |
| Age (years) | |||
| ≤65 | 47/14 | 21/35 | 0.23 (0.12, 0.42) |
| >65 | 42/13 | 14/43 | 0.20 (0.11, 0.37) |
| Race | |||
| White | 67/25 | 30/66 | 0.25 (0.16, 0.40) |
| Other | 22/2 | 5/12 | 0.08 (0.02, 0.34) |
| Region | |||
| Europe | 40/10 | 13/31 | 0.17 (0.08, 0.35) |
| USA | 49/17 | 22/47 | 0.24 (0.14, 0.42) |
HR=hazard ratio
FDA Review
What are the possible side effects?
A part of LUTATHERA is radioactive. Treatment with LUTATHERA exposes patients to radiation, and may increase the risk of lifetime radiation exposure.
LUTATHERA may cause serious side effects including low levels of blood cells, abnormal blood cell production (secondary myelodysplastic syndrome), leukemia, kidney or liver injury, increased levels of hormones in the body, (neuroendocrine hormonal crisis), injury to a fetus, and infertility.
The most common side effects of LUTATHERA are nausea, vomiting, tiredness, abdominal pain, and diarrhea.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in patients with somatostatin-receptor positive midgut GEP-NETs, who received at least one dose of LUTATHERA, in the NETTER-1 trial (safety popuation).
Table 4. Adverse Reactions Occurring in ≥ 5% (All Grades) of Patients Receiving LUTATHERA in NETTER-1
| Adverse Reaction1 | LUTATHERA and Long-Acting Octreotide (30 mg) (N = 111) |
Long-Acting Octreotide (60 mg) (N = 112) |
||
|---|---|---|---|---|
| All Grades % | Grades 3-4 % | All Grades % | Grades 3-4 % | |
| Cardiac disorders | ||||
| Atrial fibrillation | 5 | 1 | 0 | 0 |
| Gastrointestinal disorders | ||||
| Nausea | 65 | 5 | 12 | 2 |
| Vomiting | 53 | 7 | 9 | 0 |
| Abdominal pain | 26 | 3 | 19 | 3 |
| Diarrhea | 26 | 3 | 18 | 1 |
| Constipation | 10 | 0 | 5 | 0 |
| General disorders | ||||
| Fatigue | 38 | 1 | 26 | 2 |
| Peripheral edema | 16 | 0 | 9 | 1 |
| Pyrexia | 8 | 0 | 3 | 0 |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 21 | 0 | 11 | 3 |
| Musculoskeletal and connective tissue disorders | ||||
| Back pain | 13 | 2 | 10 | 0 |
| Pain in extremity | 11 | 0 | 5 | 0 |
| Myalgia | 5 | 0 | 0 | 0 |
| Neck Pain | 5 | 0 | 0 | 0 |
| Nervous system disorders | ||||
| Headache | 17 | 0 | 5 | 0 |
| Dizziness | 17 | 0 | 8 | 0 |
| Dysgeusia | 8 | 0 | 2 | 0 |
| Psychiatric disorders | ||||
| Anxiety | 12 | 1 | 5 | 0 |
| Renal and urinary disorders | ||||
| Renal failure* | 12 | 3 | 3 | 1 |
| Radiation-related urinary tract toxicity** | 8 | 0 | 3 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||||
| Cough | 11 | 1 | 6 | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Alopecia | 12 | 0 | 2 | 0 |
| Vascular disorders | ||||
| Flushing | 14 | 1 | 9 | 0 |
| Hypertension | 12 | 2 | 7 | 2 |
1National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Only displays adverse reactions occurring at a higher incidence in LUTATHERA-treated patients [between arm difference of ≥5% (all grades) or ≥2% (grades 3-4)]
*Includes the terms: Glomerular filtration rate decreased, acute kidney injury, acute prerenal failure, azotemia, renal disorder, renal failure, renal impairment
**Includes the terms: Dysuria, micturition urgency, nocturia, pollakiuria, renal colic, renal pain, urinary tract pain and urinary incontinence
The following rates of serious adverse reactions were reported in 811 evaluable patients from the ERASMUS experience: myelodysplastic syndrome (2%), acute leukemia (1%), renal failure (2%), hypotension (1%), cardiac failure (2%), myocardial infarction (1%), and neuroendocrine hormonal crisis (1%).
LUTATHERA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: Most of the patients were White. Differences in the occurrence of side effects among races could not be determined, because of the small number of patients in other races.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the most common organ system based (gastrointestinal) adverse events by subgroup in safety population from NETTER-1 trial.
Table 5. Gastrointestinal Events by Subgroup in NETTER-1 Trial (Safety Population)
| Demographic Parameters | LUTATHERA + Long-Acting Octreotide n/N (%) |
Long-Acting Octreotide n/N (%) |
|---|---|---|
| Sex | ||
| Men | 51/59 (86%) | 35/52 (67%) |
| Women | 51/53 (96%) | 41/59 (69%) |
| Age Group | ||
| <65 years="" of=""> | 55/58 (95%) | 39/54 (72%) |
| ≥65 years of age | 47/54 (87%) | 37/57 (65%) |
| Race | ||
| White | 83/88 (94%) | 67/94 (71%) |
| African American or Black | 4/5 (80%) | 2/5 (40%) |
| Asian | 1/1 (100%) | 0/0 (0%) |
| Other | 6/6 (100%) | 3/4 (75%) |
1Race and/or ethnicity was not collected for 21 patients, in France, due to local regulations
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved LUTATHERA based primarily on evidence from one clinical trial, NETTER-1 (NCT01578239) of 229 patients with somatostatin-receptor positive midgut GEP-NETs. The trial was conducted at 41 sites in Belgium, France, Germany, Italy, Portugal, Spain, United Kingdom, and the United States.
FDA also considered additional information from a large single-center experience (ERASMUS), in patients with somatostatin-receptor positive GEP-NETs other than those arising from the midgut. The patients were treated in the Netherlands. Demographics of these patients are presented in Table 7, under MORE INFO section.
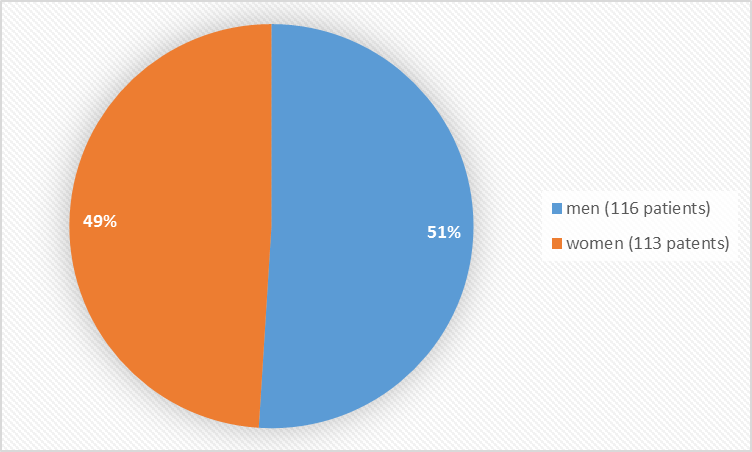
Figure 1 summarizes how many men and women were enrolled in the clinical trial NETTTER-1 used to evaluate efficacy and safety.
Figure 1. Baseline Demographics by Sex
FDA Review
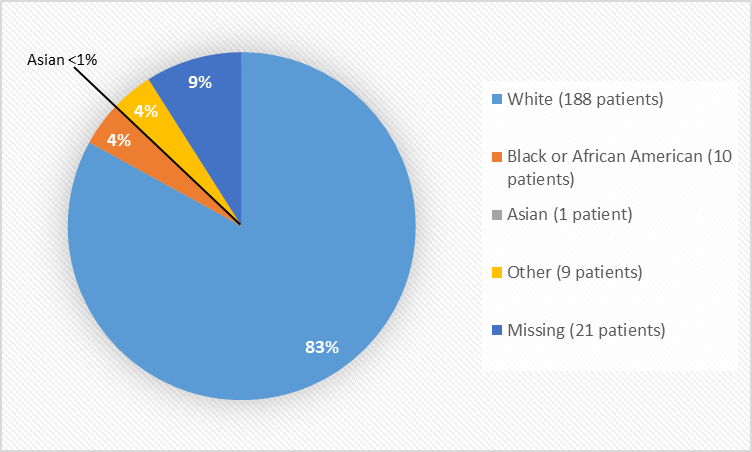
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trial NETTER-1.
Figure 2. Baseline Demographics by Race
1 Race was not collected for 21 patients, in France, due to local regulations
FDA Review
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 188 | 83% |
| Black or African American | 10 | 4% |
| Asian | 1 | less than 1% |
| Other | 9 | 4% |
| Missing1 | 21 | 9% |
1 Race was not collected for 21 patients, in France, due to local regulations
FDA Review
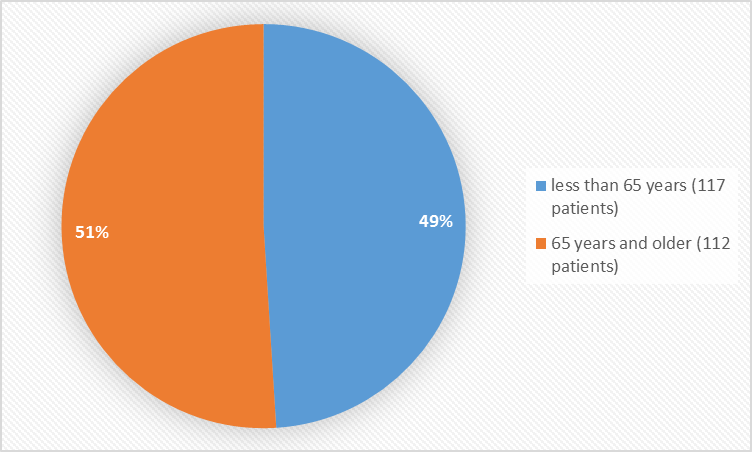
Figure 3 summarizes the percentage of patients by age in the clinical trial NETTER-1.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The tables below summarize demographics of patients enrolled in the clinical trials.
Table 6. Demographic Characteristics of Patients Enrolled in NETTER-1 Trial
| Demographic Parameters | LUTATHERA + Long-Acting Octreotide (30 mg) N=116 n (%) |
Long-Acting Octreotide (60 mg) N=113 n (%) |
Total N=229 n (%) |
|
|---|---|---|---|---|
| Sex | ||||
| Men | 63 (54%) | 53 (47%) | 116 (51%) | |
| Women | 53 (46%) | 60 (53%) | 113 (49%) | |
| Age | ||||
| Median (years) | 64 | 65 | 64 | |
| Median (range) | (28, 84) | (34, 87) | (28, 87) | |
| Age Groups | ||||
| < 65=""> | 61 (53%) | 56 (50%) | 117 (51%) | |
| ≥ 65 years | 55 (47%) | 57 (50%) | 112 (49%) | |
| Race | ||||
| White | 92 (79%) | 96 (85%) | 188 (82%) | |
| Black or African American | 5 (4%) | 5 (4%) | 10 (4%) | |
| Asian | 1 (1%) | 0 (0%) | 1 (<> | |
| Other | 6 (5%) | 3 (3%) | 9 (4%) | |
| Missing1 | 12 (10%) | 9 (8%) | 21 (9%) | |
| Ethnicity | ||||
| Hispanic or Latino | 6 (5%) | 2 (2%) | 8 (3%) | |
| Not Hispanic or Latino | 98 (84%) | 102 (90%) | 200 (87%) | |
| Missing1 | 12 (10%) | 9 (8%) | 21 (9%) | |
| Region | ||||
| North America | 66 (57%) | 69 (61%) | 135 (59%) | |
| Europe | 50 (43%) | 44 (39%) | 94 (41%) | |
1 Race and/or ethnicity was not collected for 21 patients, in France, due to local regulations
FDA Review
Table 7. Demographic Characteristics of Patients Treated on ERASMUS Protocol
| Demographic Parameters | N=1214 n (%) |
|---|---|
| Sex | |
| Men | 658 (54%) |
| Women | 556 (46%) |
| Age | |
| Median (years) | 58 |
| Median (range) | (16, 90) |
| Age group | |
| < 65=""> | 838 (69%) |
| ≥ 65 years | 376 (31%) |
| Region | |
| North America | 132 (11%) |
| Europe | 1021 (84%) |
| Latin America/Asia Pacific | 57 (5%) |
| Africa | 4 (<> |
1Data on race and/or ethnicity were not reported in the ERASMUS database
Clinical Trial Report
How were the trials designed?
The benefit and side effects of LUTATHERA were primarily evaluated in one clinical trial, NETTER-1 (NCT01578239). Enrolled patients had tumors which could not be surgically removed and were worsening while receiving treatment with octreotide.
Patients were randomly assigned to receive either LUTATHERA with long-acting octreotide or long-acting octreotide, at a higher dose, alone. LUTATHERA was injected through the vein and long-acting octreotide was injected in the muscle. Both, patients and health care providers knew which treatment was given. The benefit of LUTATHERA was evaluated by measuring the length of time that tumors did not grow after treatment and compared it to the control group (progression free survival).
The FDA considered additional data from a single-center in the Netherlands, ERASMUS. All patients received LUTATHERA with octreotide. Patients and health care providers knew which treatment was given. The benefit of LUTATHERA was evaluated by measuring if and how much the tumor size changed during treatment (the overall response rate).
How were the trials designed?
NETTER-1 was an open-label, randomized, active controlled trial in adults with progressive, well-differentiated, locally advanced/inoperable or metastatic somatostatin receptor-positive midgut carcinoid tumors. Patients were randomized to receive either LUTATHERA 7.4 GBq every 8 weeks via intravenous infusion for a maximum of 4 doses with octreotide intramuscularly 30 mg every 4 weeks, or octreotide 60 mg intramuscularly every 4 weeks. The primary efficacy outcome was progression free survival as determined by a blinded independent radiology committee (IRC) per RECIST v1. 1.
ERASMUS was a collection of data from a single center, open-label, single-arm treatment protocol of patients with midgut tumors and various somatostatin-receptor positive neuroendocrine tumors. All patients received LUTATHERA as 4 intravenous infusions of 7.4 GBq (200 mCi) at 6-13 week intervals. The primary efficacy outcome was overall response rate as evaluated by investigator using RECIST criteria.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.